Home / Rad-97 Family
Rad-97 Family
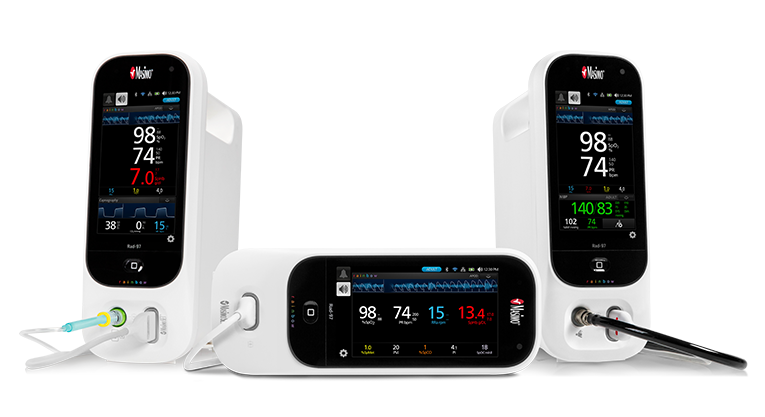
Rad-97®
Not Your Ordinary Patient Monitor
The Rad-97 Pulse CO-Oximeter®, also available with integrated NomoLine® capnography or noninvasive blood pressure (NIBP) measurement, offers advanced patient monitoring technologies in a compact, portable, and highly configurable standalone device.
Rad-97, which can be mounted on a mobile roll stand, is customisable, upgradeable, and incorporates advanced connectivity and data integration capabilities, allowing clinicians to tailor the monitor to best suit various clinical needs. Rad-97’s sleek, responsive, and intuitive multi-touch display quickly provides clinicians with pertinent data gathered from multiple advanced monitoring technologies, offering a more complete picture of a patient’s physiological status.
Advanced, Upgradeable Monitoring Technologies
Advanced, Upgradeable Monitoring Technologies
All three Rad-97 models provide noninvasive and continuous monitoring through Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry and upgradeable rainbow SET® technologies, including total haemoglobin (SpHb®) and acoustic respiration rate (RRa®).
Pulse Oximetry
Oxygen
Saturation
Pulse Rate
Perfusion Index
Pulse CO-Oximetry
Pleth Variability Index
rainbow Acoustic Monitoring®
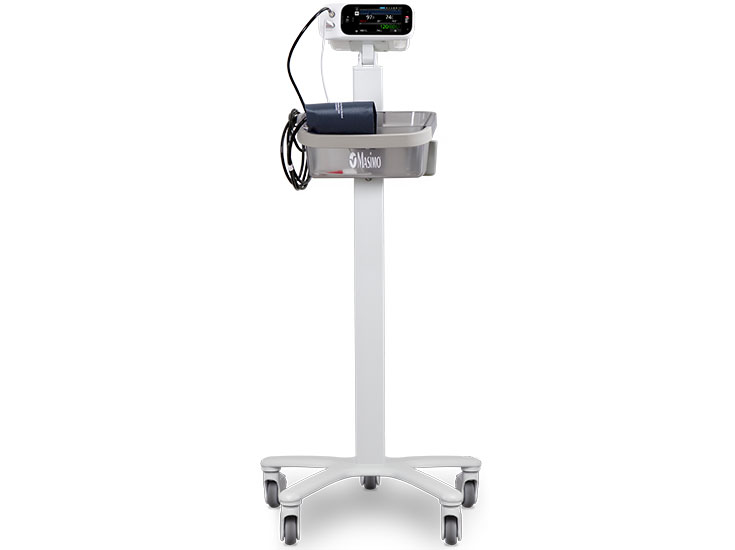
Rad-97 with NIBP provides three measurement modes of oscillometric blood pressure: spot-check, automatic interval, and stat interval. The integrated NIBP technology is compatible with both single-patient-use and reusable cuffs for adult, paediatric, and neonatal patients.

Rad-97 with NomoLine capnography features an integrated sidestream gas analyser for capnography — meeting continuous Pulse CO-Oximetry and capnography needs in a single device. This Rad-97 model provides continuous end-tidal carbon dioxide (EtCO2) monitoring with numeric, trend, and waveform viewing options, as well as fractional concentration of inspired carbon dioxide (FiCO2) and respiration rate (RR).

Rad-97 is available with the Eve™ Newborn Screening Application, which combines Masimo SET® pulse oximetry with step-by-step instructions to assist clinicians in screening for Critical Congenital Heart Disease (CCHD). Eve simplifies the CCHD screening process, per an established protocol1, by providing visual instructions, animations, an automatic synchronisation algorithm, and a detailed, easy-to-interpret display of screening results.
Integrated Connectivity Solutions for Simplified Workflows
Integrated Connectivity Solutions for Simplified Workflows
Built-in enterprise Wi-Fi capability allows Rad-97 to connect wirelessly to Masimo Patient SafetyNet™* or Iris® Gateway, facilitating automatic electronic charting of patient data to electronic medical record (EMR) systems. The continuous, automated flow of patient data from Rad-97 to patient data management systems, such as EMRs, may help reduce charting errors2 and streamline clinician workflows.
Rad-97 is also compatible with existing nurse call systems and features Ethernet and USB ports for seamless integration into wired infrastructures.

Rad-97

Patient SafetyNet/Iris Gateway

Electronic Medical Record
Highly Customisable and Adaptable for Multiple Clinical Environments
Highly Customisable and Adaptable for Multiple Clinical Environments
Rad-97 device settings can be configured to suit a variety of clinical environments, workflows, clinician preferences, and patient-specific needs.
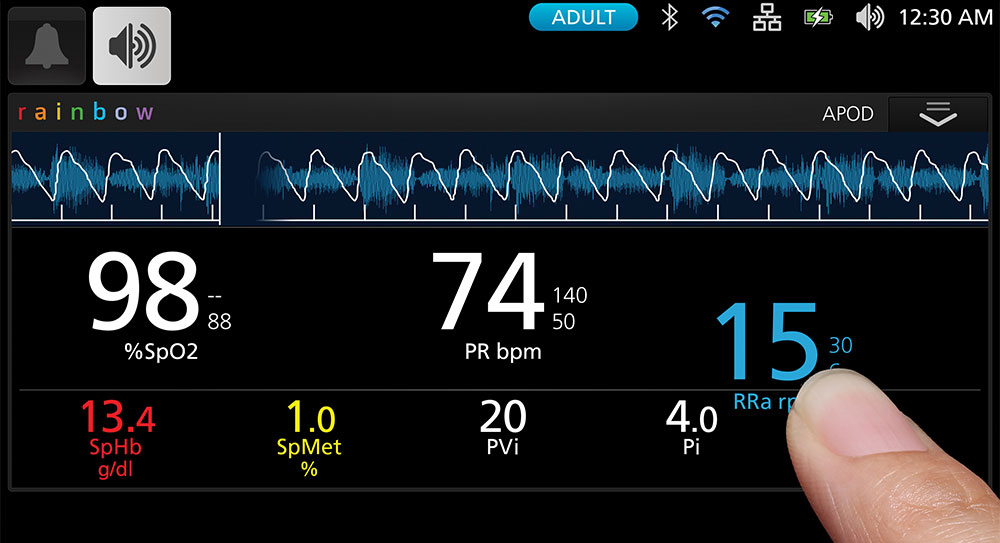
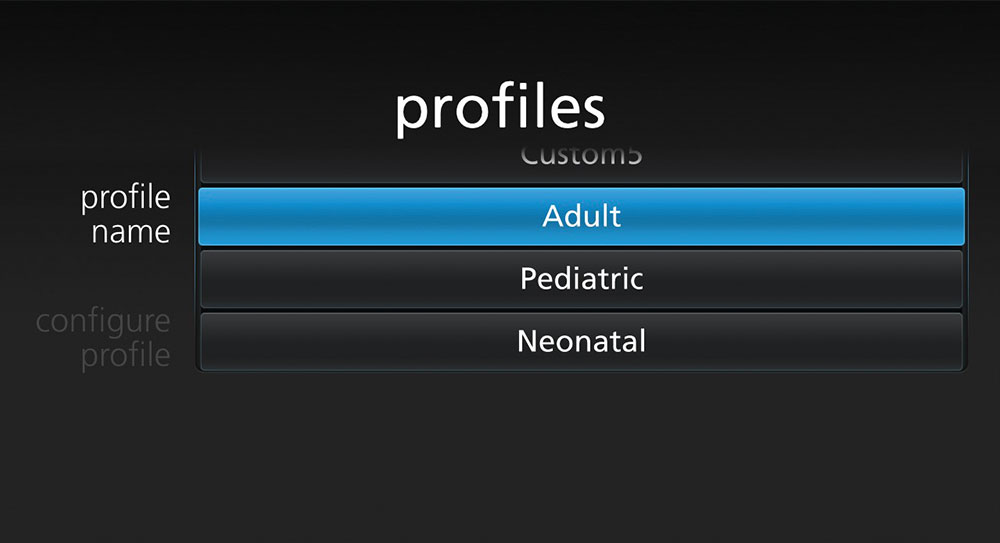
Clinicians can easily customise the high-resolution, multi-touch display to feature the most applicable measurements for each patient, with options to view parameter data at a glance in numeric view or assess patients over time in trend view. Users can also rapidly configure the device to accommodate different patient populations using pre-programmed and customisable profiles for adult, paediatric, and neonatal patients. In addition, Rad-97 can be mounted on a portable roll stand for tetherless device transport, offering flexibility in situations where space is limited.
Rad-97 Configurations
References:
*The use of the trademark PATIENT SAFETYNET is under license from University HealthSystem Consortium.
1 Kemper AR, Mahle WT, Martin GR, Cooley WC, Kumar P, Morrow WR, Kelm K, Pearson GD, Glidewell J, Grosse SD, Howell, RR. Strategies for Implementing Screening for Critical Congenital Heart Disease. Pediatrics. 2011 Oct; 128(5):1259-67.
2 The Value of Medical Device Interoperability. West Health Institute. 2013.
RESOURCES
ORi has obtained CE Marking. Not available in the U.S.
For professional use. See instructions for use for full prescribing information, including indications, contraindications, warnings, and precautions.
PLCO-003232/PLM-11162B-1019






