News & Media-2018
Home / 2018
2018
NEWS & MEDIA:

Masimo Founder and CEO Joe Kiani Honored with First IP Champion Award
The Intellectual Property Owners Education Foundation Promotes the Understanding of Intellectual Property Laws and Their Value to Society
Washington, DC – December 12, 2018 – Masimo (NASDAQ: MASI) Founder and CEO, Joe Kiani, was honored last night at the 2018 IPO Education Foundation Awards Dinner with the first ever IP Champion Award. The Intellectual Property Owners Education Foundation, a non-profit organization devoted to educating the public on the importance of IP to society, has been awarding an Inventor of the Year for 45 years and Distinguished IP Professional for 10 years. 2018 marks the creation of a new award, IP Champion, bestowed upon Mr. Kiani for showing "extraordinary leadership in advocating for the value of intellectual property to the progress of innovation."
-

Masimo Founder and CEO Joe Kiani speaking at the 2018 IPO Education Foundation Awards Dinner
Retired Chief Judge Paul Michel, formerly U.S. Circuit Judge of the U.S. Court of Appeals for the Federal Circuit and Chief Judge of that court, commented, "Serial inventor Joe Kiani built a major patient health improving company, starting from scratch at age 23, obtaining VC funding based on his patent applications when patents were strong. Later Congressional and Supreme Court interventions so sapped patent strength that he could not repeat his feat today. Reviving patents is essential to America's future since most major inventions, new jobs, and personal welfare gains and economic growth result from start-ups and small and mid-sized technology companies like Masimo. It actually saves many lives each day with its patient monitoring devices. Isn't that what we need as much today as before?"
In his acceptance speech, Mr. Kiani noted, "Without a strong IP system, Masimo would not have been able to get funding, would not have been able to stop the monopolists in our market that tried to steal it, and would not have been able to solve unsolvable problems like the development of noninvasive hemoglobin measurement. The system worked – and I want to thank the patent office and our judicial system, including the many incredible judges and juries who are able to tell truth from falsehood."
Mr. Kiani continued, "Throughout all of Masimo’s growth, we haven’t changed our philosophy nor our actions when it comes to intellectual property. We believe property rights are essential to a growing economy, and when it comes to a growing innovation economy, intellectual property rights are even more necessary. That’s why we use our success story to remind Congress, the FTC, and anyone who will listen that when it comes to IP protection, one must take the long view. . . . If we make patents and trade secrets strong, future Masimo will also be able to raise funds to solve unsolvable problems, ones hopefully even more important for our survival and economic prosperity."
The awards dinner was held on December 11th at the National Building Museum in Washington, DC. Mr. Kiani was accompanied by his wife Sarah and guests Retired Senator Tom Harkin and his wife Ruth. David Hall, Founder and CEO of Velodyne LiDAR, was named Inventor of the Year.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.3-5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
3. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
4. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

International Consensus Statement on Postoperative Anemia Management Recommends Noninvasive Hemoglobin Measurement Including Masimo SpHb®
Neuchatel, Switzerland – November 12, 2018 – Masimo (NASDAQ: MASI) announced today that a new consensus statement on postoperative anemia management was published in Anaesthesia by an international panel of clinicians specializing in patient blood management, which includes clear guidance on the use and benefits of noninvasive hemoglobin measurement.1 The statement, which discusses both spot-check and continuous measurement, references the Masimo Rad-67™ Pulse CO-Oximeter®, a spot-check device that measures noninvasive hemoglobin using Masimo SpHb®, as the example of noninvasive measurement.
-

Masimo Rad-67™ with SpHb®
The statement notes in part that, "The use of non-invasive continuous haemoglobin monitoring devices instead of phlebotomy may reduce blood loss, pain and discomfort for the patient, but concerns about precision limit routine clinical use. Although the debate focuses on accuracy of a single check, the reliability of non-invasive haemoglobin monitoring devices for dynamic changes over time may permit detection of occult bleeding and response to therapy."
In addition to this newest consensus statement, SpHb has recently received positive recognition from two other reputable institutions in the European anesthesiology community. Both the 2017 European Society of Anaesthesiology's (ESA) Guidelines for the Management of Severe Perioperative Bleeding2 and the 2017 Italian Ministry of Health's Blood Management Program Guidelines3 included noninvasive and continuous SpHb as a recommended tool for monitoring hemoglobin. Specifically, the ESA guidelines provided a strong recommendation that "continuous haemoglobin monitoring can be used as a trend monitor."
SpHb is available on a variety of Masimo noninvasive spot-check and continuous monitoring devices, both portable and bedside, as well as through licensed third-party devices. For continuous monitoring, these include Masimo’s Rad-97™, Radical-7®, and Radius-7®. Noninvasive and continuous hemoglobin (SpHb) monitoring helps automate the patient’s hemoglobin status and provides real-time visibility to changes – or lack of changes – in hemoglobin between invasive blood samples. For spot-check SpHb measurement, Masimo Pronto® is available in addition to Rad-67. Next Generation SpHb, available on these devices outside the U.S., significantly advances noninvasive hemoglobin spot-checking with improved motion tolerance, faster time to display SpHb results, and enhanced field performance in low hemoglobin ranges.
Joe Kiani, Founder and CEO of Masimo, said, "We are happy to see growing recognition, from some of the world's most renowned clinicians, institutions, and advisory bodies, of the utility and benefits of our noninvasive hemoglobin measurement technology. Studies on three continents have shown that continuous SpHb monitoring optimizes blood transfusion4-6 and in a trial with over 3,000 patients, continuous SpHb and PVi® were shown to reduce mortality 30 and 90 days after surgery.7 Never content, we continue to refine and improve SpHb and PVi, and look forward to bringing the advantages of Next Generation SpHb to additional markets and devices soon."
Rad-67 with Next Generation SpHb has not received FDA clearance and is not available in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,8 improve CCHD screening in newborns,9 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.10-12 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,13 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.14 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Munoz M, Acheson AG, Bisbe E, Butcher A, Gomez-Ramirez S, Khalafallah AA, Kehlet H, Kietaibl S, Liumbruno GM, Meybohm P, Rao Baikady R, Shander A, So-Osman C, Spahn DR, and Klein AA. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018. Doi:10.1111/anae.14358.
2. Kozek-Langenecker SA, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update 2016. Eur J Anaesthesiol. 2017 Jun;34(6):332-395. doi: 10.1097/EJA.0000000000000630.
3. Vaglio S, et al. The Italian Regulatory Guidelines for the implementation of Patient Blood Management. Blood Transfus. 2017 Jul;15(4):325-328. doi: 10.2450/2017.0060-17.
4. Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
5. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedia Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
6. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
7. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
8. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
9. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
10. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
11. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
12. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
13. Estimate: Masimo data on file.
14. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

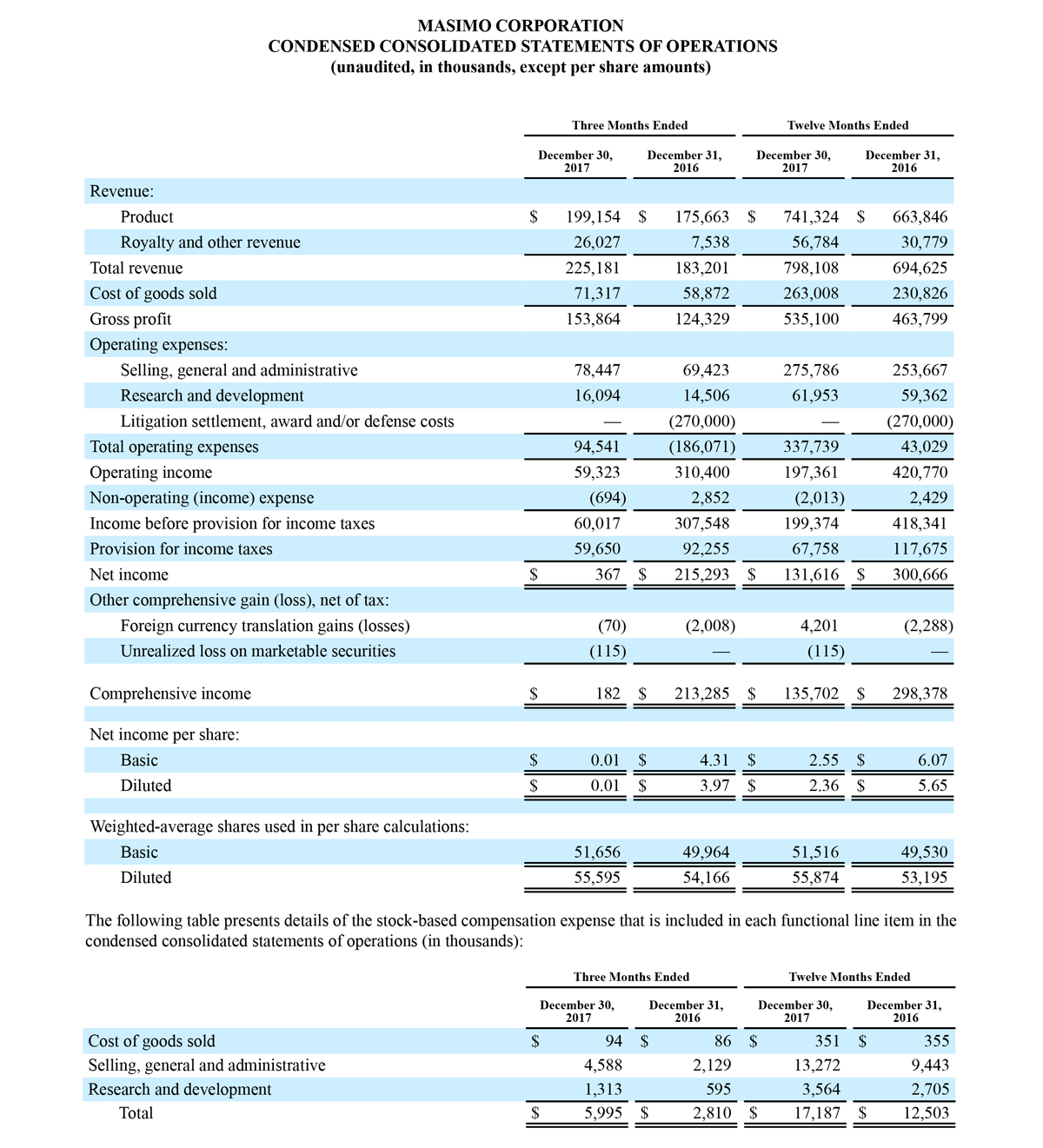
Masimo Reports Third Quarter 2018 Financial Results
Q3 2018 Highlights
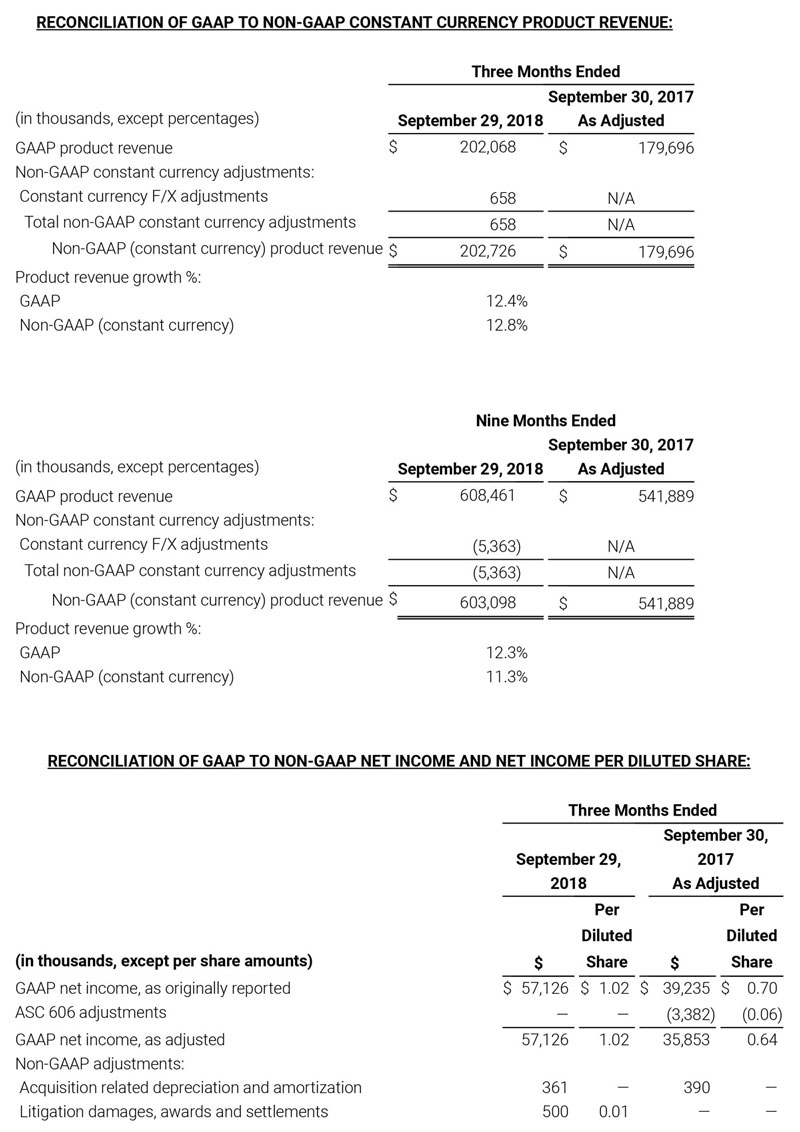
- Total revenue, including royalty and other revenue, was $210.6 million;
- Product revenue increased 12.4% to $202.1 million, or 12.8% on a constant currency basis;
- Shipments of noninvasive technology boards and monitors were 59,100; and
- GAAP net income per diluted share of $1.02. Non-GAAP net income per diluted share increased 26.8% to $0.71.
IRVINE, Calif.--(BUSINESS WIRE)--Oct. 31, 2018-- Masimo (NASDAQ: MASI) today announced its financial results for the third quarter ended September 29, 2018.
Third Quarter 2018 Results:
Third quarter 2018 total revenue, including royalty and other revenue, was $210.6 million. Product revenue for the third quarter 2018 increased 12.4% to $202.1 million, or 12.8% on a constant currency basis.
During the third quarter of 2018, the Company shipped approximately 59,100 noninvasive technology boards and monitors.
The Company’s worldwide direct product revenue, which accounted for 86.0% of total product revenue, increased to $173.8 million in the third quarter 2018. OEM sales, which accounted for 14.0% of total product revenue, increased to $28.3 million for the third quarter 2018.
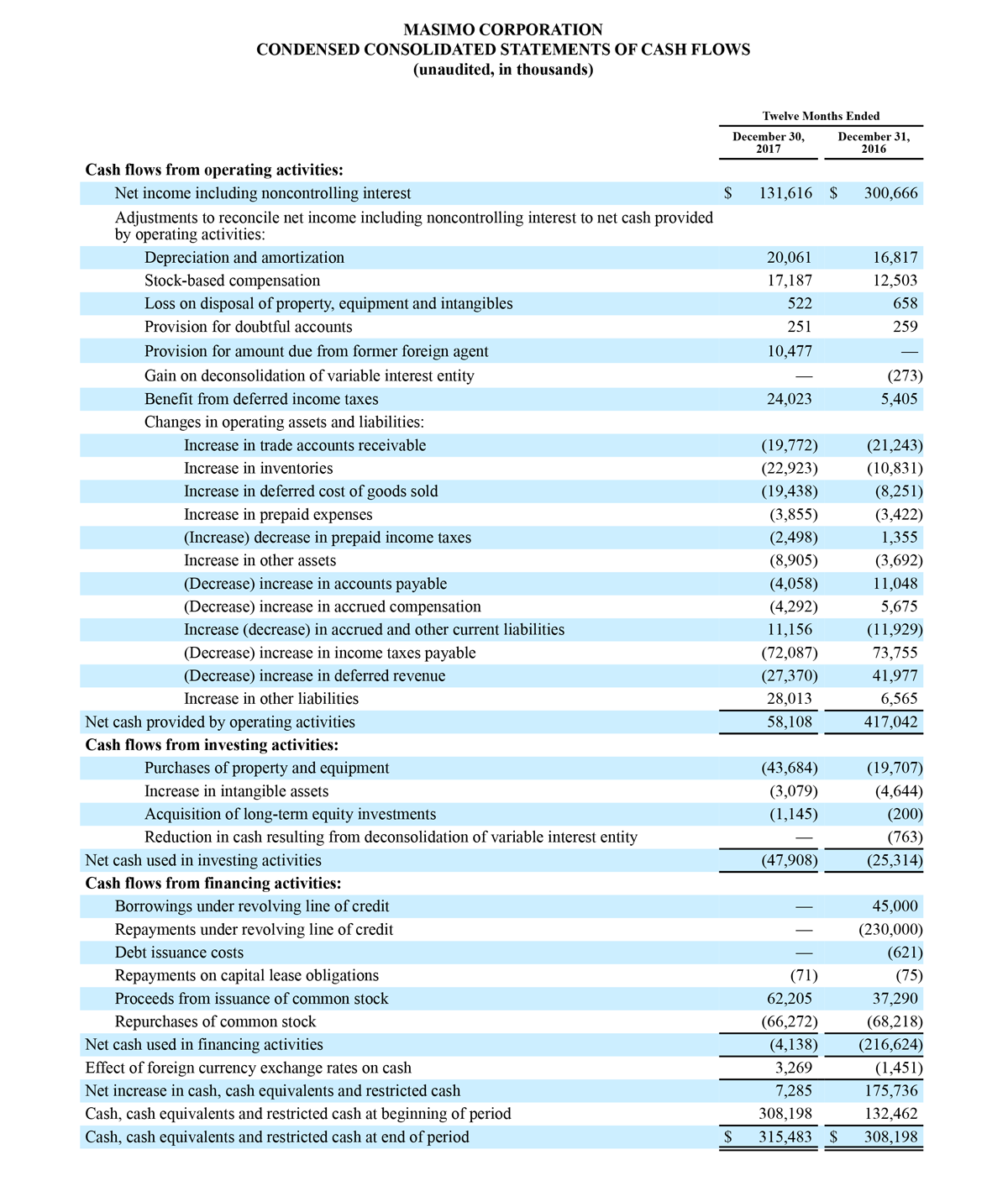
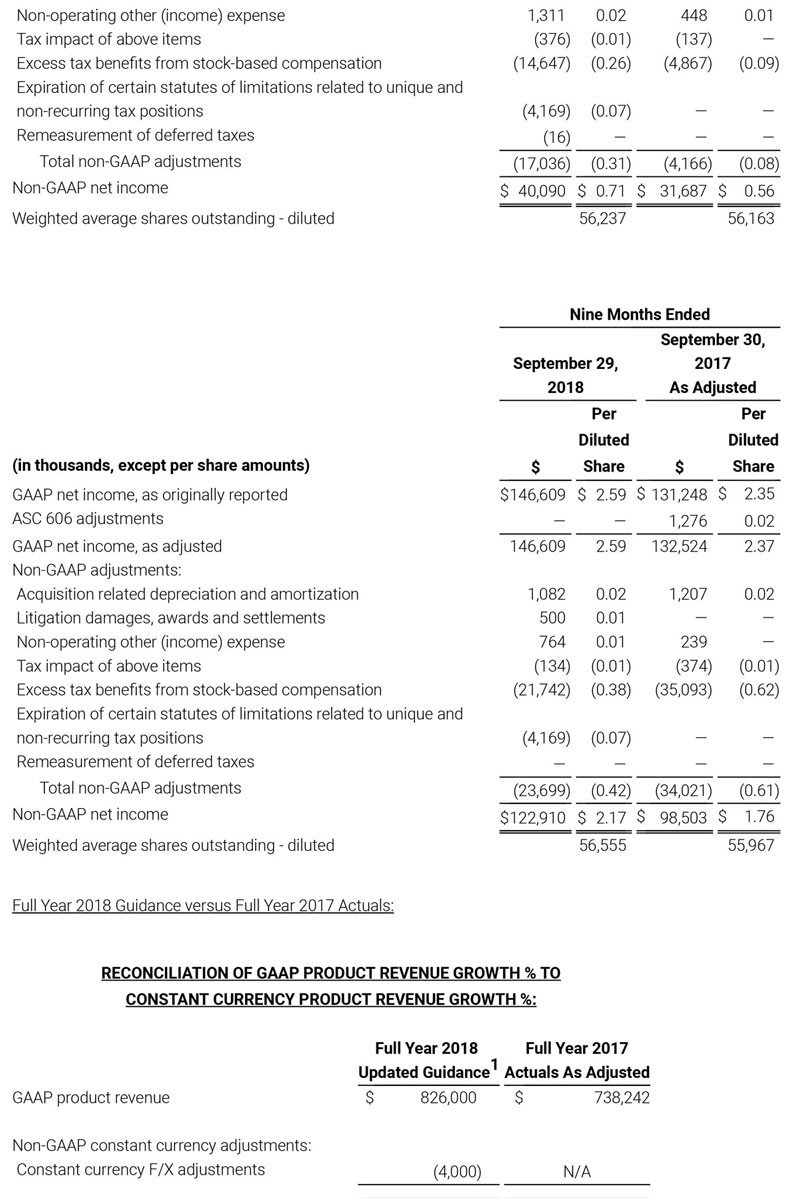
For the third quarter 2018, GAAP net income was $57.1 million or $1.02 per diluted share. Non-GAAP net income was $40.1 million, or $0.71 per diluted share.
Total cash and cash equivalents increased by $63.8 million during the quarter to $493.5 million, as of September 29, 2018.
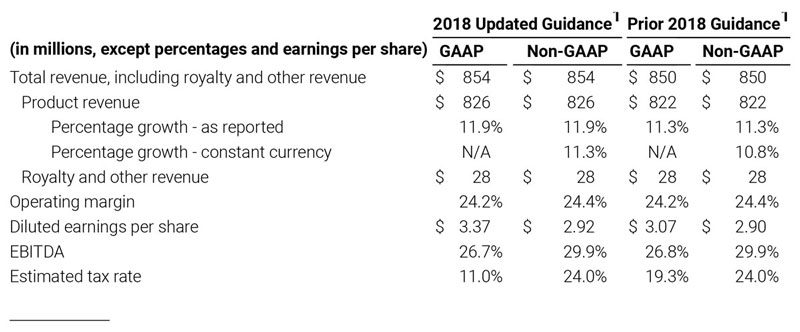
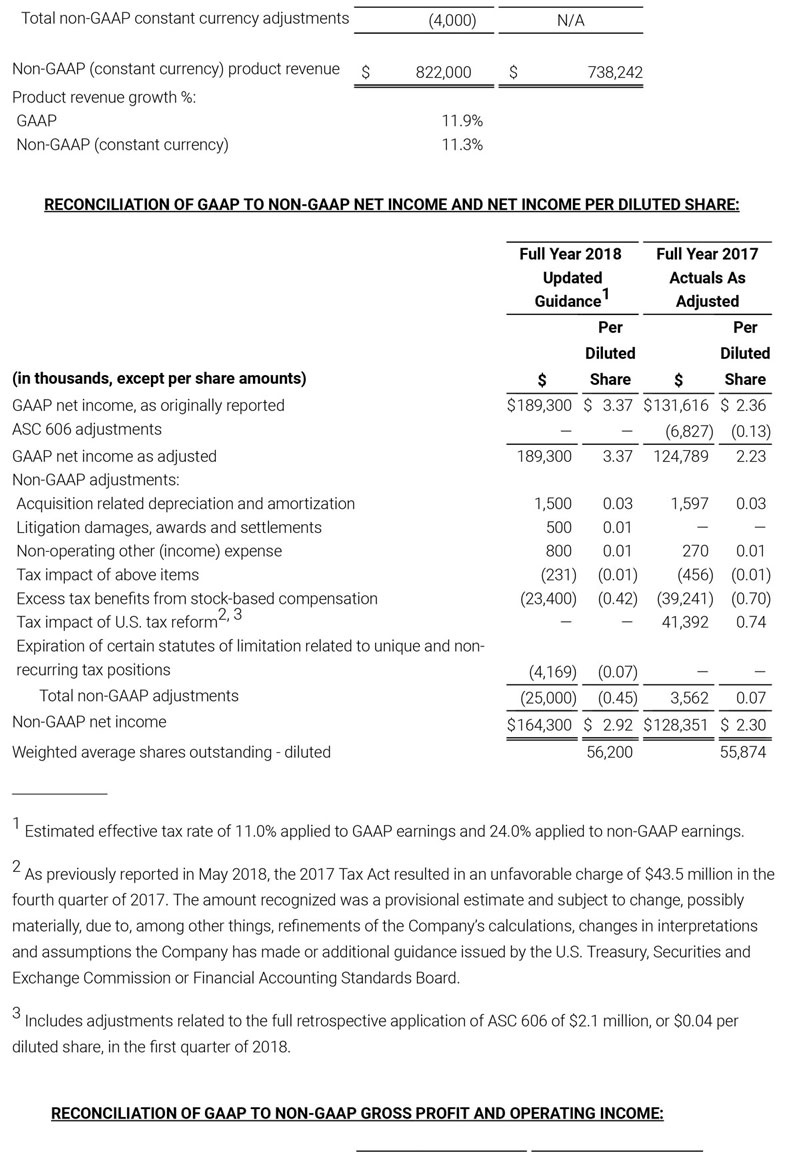
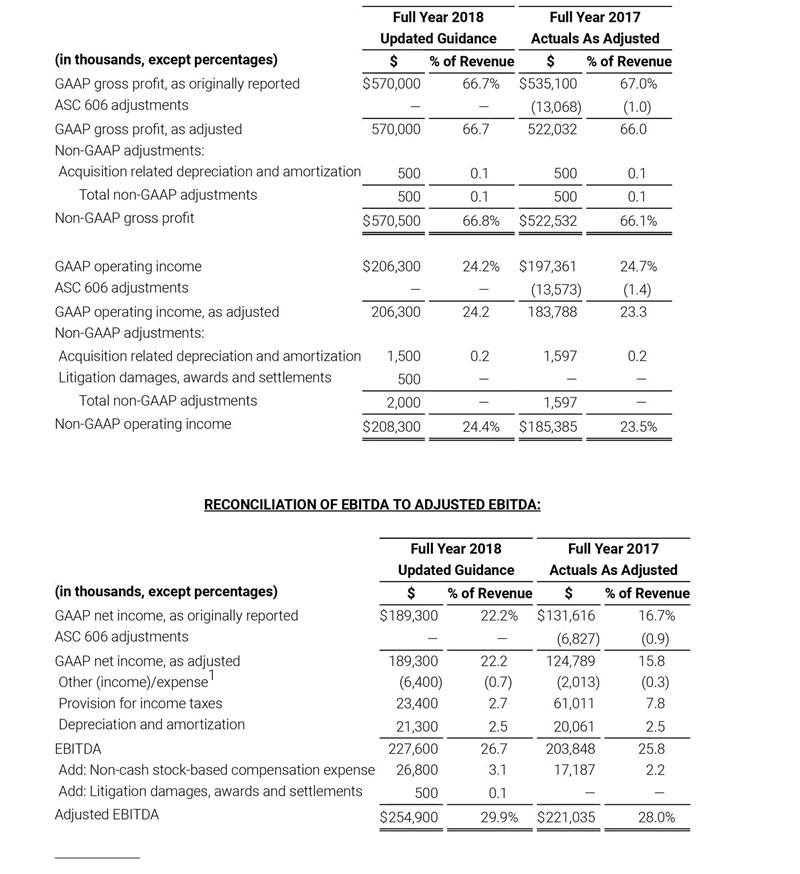
As a result of the strong performance in the third quarter, Masimo is raising its guidance for fiscal year 2018. The Company now expects product revenues of $826 million, which reflects reported growth of 11.9% and constant currency growth of 11.3%. Masimo is also raising its GAAP EPS guidance to $3.37 and its non-GAAP EPS guidance to $2.92.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, “We are happy to report results for the third quarter that exceeded expectations. Our product revenue increased 12.8% on a constant currency basis to reach $202 million for the quarter. We also shipped a record 59,100 noninvasive technology boards and monitors. Our strong growth is the result of increasing demand for our innovative technologies and systems solutions, which enable our customers to automate patient management across the continuum of care and improve patient safety. We are once again raising guidance for revenue and earnings in 2018 as we continue to grow due to our life saving and life improving technologies.”
2018 Financial Guidance
The Company provided the following updated estimates for its full year 2018 guidance:
1 Updated guidance provided October 31, 2018. Prior guidance provided August 1, 2018.
- Total revenue, including royalty and other revenue, increasing to $854 million;
- Product revenue increasing to $826 million, which reflects reported growth of 11.9% and constant currency growth of 11.3%;
- GAAP diluted earnings per share increasing to $3.37;
- Non-GAAP diluted earnings per share increasing to $2.92; and
- Included in our full year revenue guidance is approximately $4 million of year-over-year currency benefits.
Impact of Adoption of New Revenue Accounting Standard:
During the first quarter of 2018, the Company adopted Financial Accounting Standards Board (FASB) Accounting Standards Update No. 2014-09, Revenue (Topic 606): Revenue from Contracts with Customers (ASU 2014-09). The new revenue recognition standard requires the Company to make numerous assumptions that are based upon historical trends and management judgment. These assumptions may change over time and may have a material impact on our revenue recognition, guidance and results of operations. In accordance with the full retrospective method of adoption, the Company has adjusted certain amounts previously reported in its unaudited condensed consolidated financial statements to comply with the new standard, as indicated by the notation, “As Adjusted”. For additional information with respect to the impact of the adoption of this new accounting standard and reconciliations to the prior reported amounts, please reference Note 2 to our condensed consolidated financial statements that will be included in Part I, Item 1 of our Quarterly Report on Form 10-Q (Form 10-Q) for the quarter ended September 29, 2018 once filed with the Securities and Exchange Commission (SEC) and Exhibit 99.3 that was included in our Current Report on Form 8-K that was filed with the SEC today.
Supplementary Non-GAAP Financial Information
For additional non-GAAP financial details, please visit the Investor Relations section of the Company’s website at www.masimo.com to access Supplementary Financial Information.
Non-GAAP Financial Measures
The non-GAAP financial measures contained herein are a supplement to the corresponding financial measures prepared in accordance with U.S. GAAP. The non-GAAP financial measures presented exclude the items described below. Management believes that adjustments for these items assist investors in making comparisons of period-to-period operating results. Furthermore, management also believes that these items are not indicative of the Company’s on-going core operating performance. These non-GAAP financial measures have certain limitations in that they do not reflect all of the costs associated with the operations of the Company’s business as determined in accordance with GAAP.
Therefore, investors should consider non-GAAP financial measures in addition to, and not as a substitute for, or as superior to, measures of financial performance prepared in accordance with GAAP. The non-GAAP financial measures presented by the Company may be different from the non-GAAP financial measures used by other companies.
The Company has presented the following non-GAAP measures to assist investors in understanding the Company’s core net operating results on an on-going basis: (i) non-GAAP product revenue growth %, (ii) non-GAAP net income, (iii) non-GAAP diluted earnings per share, (iv) non-GAAP gross profit, (v) non-GAAP operating income and (vi) adjusted EBITDA. These non-GAAP financial measures may also assist investors in making comparisons of the Company’s core operating results with those of other companies. Management believes non-GAAP product revenue growth %, non-GAAP gross profit, non-GAAP operating income, non-GAAP net income, non-GAAP net income per diluted share and adjusted EBITDA are important measures in the evaluation of the Company’s performance and uses these measures to better understand and evaluate our business.
The non-GAAP financial measures reflect adjustments for the following items, as well as the related income tax effects thereof:
Constant currency adjustments.
Some of our sales agreements with foreign customers provide for payment in currencies other than the U.S. Dollar. These foreign currency revenues, when converted into U.S. Dollars, can vary significantly from period to period depending on the average and quarter-end exchange rates during a respective period. We believe that comparing these foreign currency denominated revenues by holding the exchange rates constant with the prior year period is useful to management and investors in evaluating our product revenue growth rates on a period-to-period basis. We anticipate that fluctuations in foreign exchange rates and the related constant currency adjustments for calculation of our product revenue growth rate will continue to occur in future periods.
Acquisition-related costs, including depreciation and amortization.
Depreciation and amortization related to the revaluation of assets and liabilities (primarily intangible assets, property, plant and equipment adjustments, inventory revaluation, lease liabilities, etc.) to fair value through purchase accounting related to value created by the seller prior to the acquisition rather than ongoing costs of operating our core business. As a result, we believe that exclusion of these costs in presenting non-GAAP financial measures provides management and investors a more effective means of evaluating historical performance and projected costs and the potential for realizing cost efficiencies within our core business. Depreciation and amortization related to the revaluation of acquisition related assets and liabilities will generally recur in future periods.
Litigation damages, awards and settlements.
In connection with litigation proceedings arising in the course of our business, we have recorded expenses as a defendant in such proceedings in the form of damages, as well as gains as a plaintiff in such proceedings in the form of litigation awards and settlement proceeds; most recently in connection with our November 2016 settlement agreement with Koninklijke Philips N.V. We believe that exclusion of these gains and losses is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these expenses and gains are generally unrelated to our core business and/or infrequent in nature.
Realized and unrealized gains or losses from foreign currency transactions.
We are exposed to foreign currency gains or losses on outstanding foreign currency denominated receivables and payables related to certain customer sales agreements, product costs and other operating expenses. As the Company does not actively hedge these currency exposures, changes in the underlying currency rates relative to the U.S. Dollar may result in realized and unrealized foreign currency gains and losses between the time these receivables and payables arise and the time that they are settled in cash. Since such realized and unrealized foreign currency gains and losses are the result of macro-economic factors and can vary significantly from one period to the next, we believe that exclusion of such realized and unrealized gains and losses are useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. Realized and unrealized foreign currency gains and losses are likely to recur in future periods.
Excess tax benefits from stock-based compensation.
Current authoritative accounting guidance requires that excess tax benefits or costs recognized on stock-based compensation expense be reflected in our provision for income taxes rather than paid-in capital. Since we cannot control or predict when stock option awards will be exercised or the price at which such awards will be exercised, the impact of such guidance can create significant volatility in our effective tax rate from one period to the next. We believe that exclusion of these excess tax benefits or costs is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. These excess tax benefits or costs will generally recur in future periods as long as we continue to issue equity awards to our employees.
Tax impacts that may not be representative of the ongoing results of our core operations.
The Tax Cuts and Jobs Act of 2017 (2017 Tax Act) was signed into law in December 2017, and became effective January 1, 2018. The 2017 Tax Act included a number of changes to existing U.S. federal tax law impacting businesses including, among other things, a permanent reduction in the corporate income tax rate from 35% to 21%, a one-time transition tax on the “deemed repatriation” of cumulative undistributed foreign earnings as of December 31, 2017 and changes in the prospective taxation of the foreign operations of U.S. multinational companies.
From time to time, we record tax benefits relating to the derecognition of uncertain tax positions due to the expiration of the statutes of limitations. During the three months ended September 29, 2018, we recorded a significant tax benefit due to the expiration of the applicable statutes of limitations related to certain non-recurring transactions.
We believe that exclusion of the tax charges related to the 2017 Tax Act and the tax benefit resulting from the expiration of certain statutes of limitations related to non-recurring transactions is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these tax items are unrelated to our core business and non-recurring in nature.
Third Quarter 2018 Actuals versus Third Quarter 2017 Actuals:
1 Other (income)/expense consists primarily of interest (income)/expense and net foreign currency (gains)/losses.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be available online from the investor relations page of the Company’s website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 2368156. After the live webcast, the call will be available on Masimo’s website through November 28, 2018. In addition, a telephonic replay of the call will be available through November 7, 2018. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 2368156.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the Company debuted Masimo SET® Measure-through Motion and Low Perfusion® pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®) and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate and perfusion index (PI). In 2014, Masimo introduced Root™, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface. Masimo is also taking an active leadership role in mobile health applications (mHealth) with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year GAAP and non-GAAP 2018 total, product, royalty and other revenues, earnings per diluted share, operating margin, EBITDA, and estimated tax rate, and our long-term outlook; demand for our products; anticipated revenue and earnings growth; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management’s current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow SET™ products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors’ assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the “Risk Factors” section of our most recent periodic reports filed with the Securities and Exchange Commission (“SEC”), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI and ORI are trademarks or registered trademarks of Masimo Corporation.

View source version on businesswire.com: https://www.businesswire.com/news/home/20181031005802/en/
Source: Masimo
Masimo
Investor Contact: Eli Kammerman
(949) 297-7077
ekammerman@masimo.com
or
Media Contact: Irene Paigah
(858) 859-7001
irenep@masimo.com

Masimo Announces FDA Clearance for Masimo RD Sensors with Improved Accuracy Specifications for SET® Pulse Oximetry
Irvine, California – October 25, 2018 – Masimo (NASDAQ: MASI) announced today that RD SET™ sensors with Masimo Measure-through Motion and Low Perfusion™ SET® pulse oximetry have received FDA clearance with improved SpO2 accuracy specifications for all patients > 3 kg. RD SET single-patient-use sensors with the improved accuracy specifications are now available. The new RD SET sensors' SpO2 accuracy specifications during patient motion have improved for adult, pediatric, and infant patients to 1.5% (at 1 SD), compared to previous accuracy specifications of 3%.
-
Masimo Radical-7® with RD SET™ Sensor and SET® Pulse Oximetry
In addition to offering improved accuracy, RD SET sensors are designed to enhance patient comfort, optimize clinician workflows, and help hospitals meet green initiatives. The sensors are lightweight and have a flat, soft cable with smooth edges, so that they lie comfortably on a patient’s hand or foot. The sensors feature an intuitive sensor-to-cable connection. Their lightweight design results in up to 84% less waste, and their sleek, recyclable packaging reduces storage and shipping space.
The clinical benefits of Masimo's revolutionary SET® pulse oximetry have been demonstrated in a variety of clinical conditions. Studies have shown:
- Masimo SET® helped significantly reduce rates of severe retinopathy of prematurity (ROP) and the need for laser treatment.1
- Masimo SET® enabled pulse oximetry screening for critical congenital heart disease (CCHD), helping save many full-term newborns' lives whose disease would otherwise have gone undiagnosed.2-4
- With Masimo SET® on post-surgical wards, rescue calls and ICU transfers were reduced by 65% and 48%, respectively.5 Post-operative continuous surveillance monitoring, combined with Masimo SET®, led to zero preventable deaths or brain damage due to opioids over five years.6
Joe Kiani, Founder and CEO of Masimo, said, "We're delighted to be able to announce our continued innovation in our foundational SET® pulse oximetry. Thanks to the brilliance and dedication of our engineers and the continuing support of our customers, we've been able to once again raise the standard for pulse oximetry performance. Even though no one has been able to create pulse oximetry that outperforms SET®, we have not allowed that to stop us from continuing our pursuit of perfecting the technology. We have significantly improved our accuracy during motion and this is just the start of further improvements in what clinicians can expect from pulse oximetry."
In addition to excellent accuracy and reliability, the SET® platform with rainbow® is also the only oximetry technology that also allows clinicians to measure physiological parameters such as total hemoglobin, carboxyhemoglobin, methemoglobin, and PVi®.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.5-7 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo et al. Prevention of retinopathy of prematurity in preterm infants through changes in clinical practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. Zhao et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
3. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4. Ewer A et al. Pulse Oximetry Screening for Congenital Heart Defects in Newborn Infants (Pulseox): A Test Accuracy Study. Lancet. 2011 Aug 27;378(9793):785-94.
5. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
6. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
7. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
8. Estimate: Masimo data on file.
9. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET® and RD SET™ sensors. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET® and RD SET sensors, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Bassett Medical Center of New York Adopts Masimo Patient SafetyNet™
and Root® with Vital Signs Check
Cooperstown, New York and Irvine, California – October 17, 2018 – Masimo (NASDAQ: MASI) announced today that Bassett Medical Center, a 180-bed Cooperstown, New York hospital, has adopted Masimo Patient SafetyNet™ and Root® with Vital Signs Check across its 54-bed medical-surgical unit, including pediatric beds. The integrated system, extending from bedside patient monitoring and data collection to supplemental remote monitoring and notification, provides advanced monitoring, streamlines workflows, automates the integration and transfer of patient data, and keeps clinicians abreast of pertinent changes in patient condition even from afar, helping to enhance quality of care and patient safety.
Ronette Wiley, RN, COO of Bassett Medical Center, commented, "We value this partnership with Masimo. Patient SafetyNet and the integrated Vital Signs Check capabilities of Root are already proving to be a great fit for our medical-surgical units. It’s been just over a month since implementation, and already the feedback from our clinicians is positive."
-
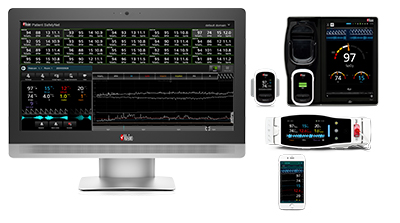
Masimo Patient SafetyNet™, Root®, Radius-7®, Radical-7®, and Replica™
In discussing several potential patient events to which their newly installed Root and Patient SafetyNet system alerted clinicians, Charles Oliver, RN, Assistant Nurse Manager at Bassett Medical Center, said of Patient SafetyNet, "This has undoubtedly prevented rapid responses, transfers and possibly even a code situation today."
Masimo Root is a powerful, expandable bedside platform that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions – in a single, clinician-centric hub. Root’s plug-and-play expansion capabilities allow clinicians to simplify patient monitoring by bringing together advanced rainbow SET™ Pulse CO-Oximetry, brain function monitoring, regional oximetry, capnography, and vital signs measurements on an easy-to-interpret, customizable display, empowering clinicians with important information for making patient assessments. Vital Signs Check for Root allows clinicians to streamline and automate vital signs measurement and patient data workflows.
Masimo Patient SafetyNet is a supplemental remote monitoring and clinician notification system which allows patient monitoring data, gathered and integrated at the bedside by Root and other Masimo devices, to be accessed from central viewing stations. When changes occur in measured values that may indicate deterioration in a patient’s condition, Patient SafetyNet automatically sends wireless alerts directly to clinicians, wherever they may be, allowing clinicians to respond quickly to patients in potential distress. In addition, Patient SafetyNet automates the transfer of patient data, including vital signs, early warning scores (EWS), and other physiological parameters, directly to hospital electronic medical record (EMR) systems, like the Epic EMR used by Bassett Medical Center.
In a landmark study at Dartmouth-Hitchcock Medical Center in New Hampshire, researchers found that continuous monitoring of adult post-surgical patients using Masimo SET® pulse oximetry on Masimo bedside devices, in conjunction with Masimo Patient SafetyNet, resulted in a 65% reduction in rapid response team activations and a 48% reduction in transfers back to the ICU.1 Over five years, they achieved their goal of zero preventable deaths or brain damage due to opioids,2 and over ten years, they maintained a 50% reduction in unplanned transfers and a 60% reduction in rescue events, despite increase in patient acuity and occupancy.3
Joe Kiani, Founder and CEO of Masimo, said, "We applaud Bassett Medical Center’s prioritization of patient safety and are happy they are already seeing the benefits of implementing integrated Masimo systems like Root and Patient SafetyNet, designed to improve and automate care. With a solid foundation of Masimo monitoring technologies like Measure-through Motion and Low Perfusion™ SET® pulse oximetry and our advanced rainbow® parameters, and the streamlined workflows made possible by Masimo's enhanced connectivity and data integration solutions, clinicians at Bassett can remain focused more acutely than ever on delivering the best possible patient care."
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Taenzer A et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
2. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
3. McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Root® and Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root and Patient SafetyNet, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

New Study Evaluates the Ability of Masimo ORi™ to Help Clinicians Reduce Hyperoxemia in Mechanically Ventilated ICU Patients
Neuchatel, Switzerland – October 1, 2018 – Masimo (NASDAQ: MASI) announced today the findings of an abstract presented at the 2018 Congress of the French Society of Anesthesia and Resuscitation (SFAR) in Paris in which researchers at CHU Angers in France investigated the ability of Masimo ORi™ (Oxygen Reserve Index) to help clinicians reduce the number of days ICU patients experience hyperoxemia while on mechanical ventilation.1 ORi, available outside the U.S., is a noninvasive and continuous parameter intended as a relative indicator of a patient’s oxygen reserve during moderate hyperoxia (partial pressure of oxygen in arterial blood [PaO2] in the range of 100 to 200 mmHg). ORi can be trended and has optional alarms to notify clinicians of changes in oxygenation.
-
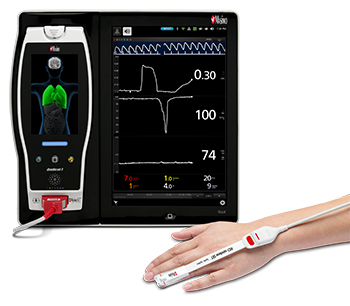
Masimo Root® with Radical-7®, ORi™, and the RD rainbow SET™ Sensor
Noting that hyperoxemia can lead to patient morbidity and mortality in ICU patients, and that oxygen saturation (SpO2) cannot detect hyperoxemia, Dr. Brochant and colleagues sought to evaluate whether ORi might be useful in helping clinicians determine when to reduce the fraction of inspired oxygen (FiO2) during oxygen therapy, so as to avoid hyperoxemia. In this initial analysis, the first 131 patients in the study, whose data were collected between May 2017 and March 2018, were randomly assigned into an ORi and a control group. In the ORi group, FiO2 was reduced if ORi was > 0. In the control group, the FiO2 level was adjusted according to SpO2. Clinicians recorded blood gas results, episodes of atelectasis, and the length of time spent on mechanical ventilation, for up to 28 days. The principal point of comparison was the proportion of ventilated days with hyperoxemia.
The researchers found that the percentage of days with hyperoxemia was significantly lower in the ORi group: median 14% [interquartile range 0-31%], vs. 29% [IQR 11-50%] in the control group, p=0.005. Average daily PaO2 and FiO2 values were not significantly different between the two groups, suggesting that FiO2 was not systematically lowered in the ORi group. The average number of days without ventilation and median time spent in the ICU were also not significantly different.
The researchers concluded that the use of ORi may help clinicians reduce the percentage of days with hyperoxemia, that analysis of the full group of patients may allow assessment of its effect on the occurrence of atelectasis, and that additional studies may be useful in evaluating the impact of this monitoring on the morbidity and mortality of patients.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
Reference
1. Brochant A, Dupre P, Gaillard T, Lemarie P, Gergaud S, and Lasocki S. ORi pour moins d’hyperoxie en reanimation. Proceedings from SFAR 2018, Paris, France. #R244.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo ORi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo ORi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces FDA Clearance of the rainbow Acoustic Monitoring® RAS-45 Sensor for Infant and Neonatal Patients
Irvine, California – September 10, 2018 – Masimo (NASDAQ: MASI) announced today FDA clearance of RAS-45, an acoustic respiration sensor for rainbow Acoustic Monitoring® (RAM®), for infant and neonatal patients. RAM could previously be used to monitor adult and pediatric patients greater than 10 kg using RAS-125c and RAS-45 sensors. With clearance of the RAS-45 sensor for infant and neonatal patients, acoustic respiration rate measurement is now, for the first time, possible for patients of all sizes, including neonates, in the United States.
-
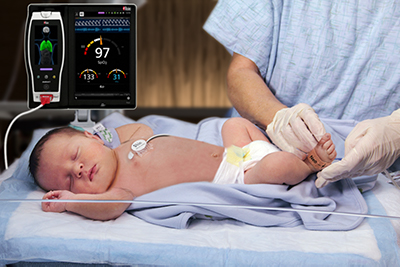
Masimo Root® with Radical-7®, RRa®, and the RAS-45 Infant/Neonatal Sensor
RAM noninvasively and continuously measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer, the RAS-45 and RAS-125c, applied to the patient’s neck area or, for infant and neonatal patients under 10 kg, the chest. Using acoustic signal processing that leverages Masimo Signal Extraction Technology® (SET®), the respiratory signal is separated and processed to display continuous respiration rate (RRa®) and an acoustic respiration waveform, a visualization of the vibrations caused by the patient’s airflow. The acoustic sensor also allows clinicians to listen to the sound of a patient’s breathing, whether at the bedside, through a point-of-care device like the Radical-7® Pulse CO-Oximeter®, or remotely, from a Patient SafetyNet™ view station.
The RAS-45 sensor for infant and neonatal patients offers multiple benefits of particular importance for successfully monitoring these youngest and most fragile patients. With the clearance for newborns and neonates, RRa’s accuracy range has been expanded up to 120 breaths per minute, while still providing accuracy of ± 1 breath per minute, facilitating accurate measurement of the higher respiratory rates common in this population. The sensor itself is significantly smaller than the RAS-125c sensor, and in fact with a diameter of approximately 2.2 cm without adhesive is only slightly larger than a nickel. Similarly, it weighs so little, 13 grams, that its presence may be barely noticeable, and features an adhesive that is transparent, light, and flexible. The size, weight, and adhesive advantages make it particularly suitable for the smaller stature and delicate skin of infants and neonates.
RRa has been shown not only to be accurate1,2 and reliable1, but also easy-to-use1, easy-to-tolerate1,3, and to enhance patient compliance with respiration monitoring. In a study comparing pediatric patient tolerance of sidestream capnography with a nasal cannula to respiration rate monitoring with an RAS-125c acoustic sensor, 15 out of 40 patients removed the capnography cannula, while only one removed the RAM acoustic sensor.3 In a study of 98 patients consciously sedated during upper gastrointestinal endoscopy, researchers found that RRa monitoring with the RAS-125c sensor more accurately assessed respiration rate than impedance pneumography.2
Joe Kiani, Founder and CEO of Masimo, commented, “From the beginning, we have focused our R&D on neonates and children for many reasons, including our belief that helping clinicians care for children will provide more benefit to society. RAM harnesses the power of our breakthrough signal processing and sensor technology and applies it to a measurement that has either been unreliable or difficult to use, respiration measurement, the third vital sign.”
RAM is available on most rainbow SET™-ready platforms. Continuous monitoring of respiration rate can be helpful in cases such as sedation-based procedures and post-surgical patients receiving patient-controlled analgesia for pain management.4,5
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Macknet MR et al. Accuracy and Tolerance of a Novel Bioacoustic Respiratory Sensor in Pediatric Patients. Anesthesiology. 2007;107:A84 (abstract).
2. Goudra BG et al. Comparison of Acoustic Respiration Rate, Impedance Pneumography and Capnometry Monitors for Respiration Rate Accuracy and Apnea Detection during GI Endoscopy Anesthesia. Open J Anesthesiol. 2013;3:74-79.
3. Patino M et al. Accuracy of Acoustic Respiration Rate Monitoring in Pediatric Patients. Paediatr Anaesth. 2013 Sep 3.
4. Stoelting, RK et al. APSF newsletter. 2011. www.apsf.org.
5. The Joint Commission Sentinel Event Alert. Issue 49, August 8, 2012. www.jointcomission.org.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo RAS-45, RAM®, and RRa®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo RAS-45, RAM, and RRa, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Compares Two Methods of Guiding Goal-Directed Fluid Therapy, Noninvasive Masimo PVi® and Invasive Esophageal Doppler
Neuchatel, Switzerland – September 5, 2018 – Masimo (NASDAQ: MASI) announced today the findings of a study recently published in BMC Anesthesiology in which researchers at University Hospital Linköping, Sweden compared the performance of goal-directed fluid therapy (GDFT) using invasive esophageal Doppler to GDFT using Masimo PVi® (pleth variability index, measured noninvasively and continuously using SET® pulse oximetry sensors) in patients undergoing major abdominal surgery.1
Noting the value of GDFT in aiming to determine “the optimal amount of fluid for an individual patient,” Dr. Hans Bahlmann and colleagues sought to compare the effects of two methods of GDFT, stroke volume optimization guided by invasive esophageal Doppler (control group) and fluid optimization guided by noninvasive PVi (intervention group), on patients who were scheduled for open abdominal surgery lasting two or more hours. The effects compared were incidence of complications and length of hospital stay.
The researchers collected data from 146 patients who had surgery between November 2011 and January 2015, who were randomly divided into the two groups. In the intervention group, PVi was noninvasively and continuously measured with a Masimo Radical-7® Pulse CO-Oximeter® with software version 7.8.0.1 and SET® sensors. After an initial fluid bolus (given to all patients irrespective of PVi value), a fluid bolus was given if PVi was ≥ 10%, repeated at 5 minute intervals until PVi fell below 10% or did not decrease at all. In the control group, esophageal Doppler measurements were performed using a Deltex Medical CardioQ apparatus. After an initial fluid bolus, Doppler measurement was performed after 5 minutes and fluid boluses were repeated until stroke volume did not increase by 10%, in accordance with published protocols.
Observers blinded to each patient’s group assessed complications for 30 postoperative days using a pre-specified list of complications, as well as length of hospital stay. The researchers found that noninvasive PVi and invasive Doppler performed with no statistical difference, with similar incidences of complications and lengths of stay between the two groups. In the intervention/PVi group, there were 64 complications (corresponding to 51% of patients), with a median length of stay of 8.0 days (interquartile range [IQR] 8.0 days). In the control/Doppler group, there were 70 complications (corresponding to 49% of patients), with a median length of stay of 8.0 days (IQR 9.5).
The researchers concluded, “No difference in clinical outcome, as defined by number of postoperative complications, and length of hospital stay, was found when goal directed fluid therapy was applied using PVI as an alternative to esophageal Doppler. PVI appears to be an acceptable alternative to esophageal Doppler for goal directed fluid therapy during major open abdominal surgery.”
In discussing other PVi-related clinical studies, the researchers also noted that “Based on these reports and our findings, a clinician wishing to pursue GDFT can choose PVI over esophageal Doppler in the large majority of patients undergoing major open abdominal surgery. PVI, in contrast to esophageal Doppler, is not sensitive to interference from diathermy and does not require frequent access to the patient’s head for probe repositioning. Also, PVI can be measured without single-use equipment avoiding the cost for single-use esophageal probes (USD 130 in our setting). However, as illustrated by the request for Doppler data in two PVI patients, the clinician might still want to have access to a reliable method for measuring intraoperative cardiac output to increase the amount of hemodynamic information during unusually complex situations or in cases of known vascular and/or myocardial dysfunction.”
As a study limitation, the researchers noted the absence of a control group with neither PVi nor Doppler guiding fluid therapy, and therefore “it is not possible to tell whether PVi and Doppler both result in similar improvements in outcome, or whether neither method improves outcome when compared to treatment without GDFT.” They also noted that because of determining sample size using a 10% difference in the number of complications at 30 days, “the study was not powered to detect smaller albeit still significant differences in postoperative outcome.”
PVi has not been cleared by the FDA to assess fluid responsiveness.
Reference
1. Bahlmann H, Hahn R, and Nilsson L. Pleth variability index or stroke volume optimization during open abdominal surgery: a randomized controlled trial. BMC Anesthesiology. (2018) 18:115. https://doi.org/10.1186/s12871-018-0579-4.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo ORi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo ORi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Evaluates the Ability of Masimo ORi™ to Reflect Oxygenation
During Moderate Hyperoxia
Neuchatel, Switzerland – August 27, 2018 – Masimo (NASDAQ: MASI) announced today the findings of a study recently published in Anesthesia & Analgesia in which researchers at the University of Groningen, the Netherlands and Ghent University, Belgium investigated the ability of Masimo ORi™ (Oxygen Reserve Index) to show trends in oxygenation in the moderate hyperoxic region (partial pressure of oxygen in arterial blood [PaO2] in the range of 100 to 200 mmHg).1 ORi is available outside the U.S. and is intended as a noninvasive, relative indicator of a patient’s oxygen reserve during moderate hyperoxia. ORi can be trended and has optional alarms to notify clinicians of changes in oxygenation.
-
Masimo Root® with Radical-7® and ORi™
Noting that monitoring oxygenation using pulse oximetry alone "gives little information on PaO2" during conditions of normoxia and hyperoxia, Dr. Jaap Jan Vos and colleagues sought to evaluate the correlation of ORi to PaO2 during moderate hyperoxia. They collected data from 20 healthy adult volunteers, who breathed a series of standardized oxygen concentrations ranging from mildly hypoxic (inspired oxygen = 14%) to extremely hyperoxic (inspired oxygen = 100%). ORi and SpO2 were noninvasively and continuously measured by sensors on both the second and fourth fingers and displayed on a Masimo Radical-7® Pulse CO-Oximeter®. At baseline and at each induced oxygenation stage, arterial blood samples were taken for blood gas analysis and PaO2 was measured invasively using a Siemens Rapidpoint 405 CO-Oximeter.
The researchers collected 1090 paired data points of simultaneous ORi and PaO2 values. In the "ORi-sensitive" range of 100-200 mmHg, the mean ORi value was 0.16, with associated oxygen saturation (SpO2) values of ≥ 97% at all points. Correlation between ORi and PaO2 was positive at all points (p < 0.0001), with R values of 0.78, 0.83, and 0.84 for sensor 1, sensor 2, and mean of both sensors, respectively. To assess ORi's trending ability, the researchers used a 4-quadrant plot and calculated that ORi trending of PaO2 within the range of 100-200 mmHg had a concordance rate of 94%.
The researchers concluded, “In this prospective volunteer validation study, a strong and positive correlation between PaO2 and ORi was found, together with a good trending ability. Based on these data, the future use of ORi as a continuous noninvasive monitoring tool for assessing oxygenation status in patients receiving supplemental oxygen might be supported.” They also noted that “in healthy volunteers, ORi provides reasonable trending information of PaO2 around the moderate hyperoxic range of PaO2 for which its use is intended. Also, changes in PaO2 are well reflected by changes in ORi, with good concordance. The trend in ORi can be used to track changes in PaO2 levels in the moderate hyperoxic region, and absolute values should not be interpreted for PaO2 levels.”
The researchers noted several limitations of the study, including that "additional studies are required to confirm these findings in a clinical setting" and that the influence of factors such as patient comorbidity and clinical circumstances requires further research. In addition, the researchers observed differences between the absolute values of simultaneously measured ORi values from sensors placed at different sites on the subject, which may require further studies in order to clinically rely on absolute ORi values as a direct measure of oxygen reserve, especially in situations where accurate oxygenation assessment may be necessary.
Study co-author Professor Thomas Scheeren commented, "ORi fills a gap in the monitoring of patients receiving supplemental oxygen by noninvasively and continuously trending the course of arterial oxygen tension. It may help to better titrate oxygen therapy to avoid both hypoxia and unintended hyperoxia."
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
Reference
1. Vos JJ, Willems CH, Van Amsterdam K, van den Berg J, Spanjersberg R, Struys MMRF, and Scheeren TW. Oxygen Reserve Index: Validation of a New Variable. Anesth Analg. 22 Aug 2018. DOI: 10.1213/ANE.0000000000003706.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo ORi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo ORi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Investigates the Utility of Home Monitoring Using Masimo SET® Pulse Oximetry to Screen Children with Down Syndrome for Risk of Obstructive Sleep Apnea Prior to Diagnostic Multichannel Sleep Studies
Southampton, United Kingdom – August 13, 2018 – Masimo (NASDAQ: MASI) announced today the findings of a recently published study in which researchers investigated whether home pulse oximetry monitoring might be a useful initial screening method of determining which children with down syndrome (DS) – who are at high risk of obstructive sleep apnea (OSA) – be recommended to undergo multichannel sleep studies to diagnose the condition. The home monitoring was conducted using Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry.1
-
Masimo Radical-7® Pulse CO-Oximeter®
Noting that OSA "can only be reliably diagnosed using multichannel sleep studies, which are expensive, demanding for families and only available in specialist centres," Dr. Hill and colleagues at the University of Southampton and Southampton Children’s Hospital sought to determine whether home pulse oximetry monitoring could identify children at high risk of OSA, and in particular which parameters could most sensitively detect this risk, as an initial screening step. To that end, they studied 161 children with DS, aged 0.5 to 6 years, of whom 25 were separately diagnosed with OSA. The patients were monitored overnight using Masimo Radical-7® Pulse CO-Oximeters®, with pulse oximetry sensors placed on the great toe. Recorded measurements included: total artifact-free time analyzed, mean oxygen saturation (SpO2), minimum SpO2, 3% oxyhemoglobin desaturation index (ODI), delta 12s index (the absolute difference between successive 12 second interval recordings, a measure of baseline SpO2 variability) and the number of minutes per hour that SpO2 was below 90%.
Receiver operating curves (ROC) and area under the curve (AUC) statistics were calculated to determine which measurements, alone and in combination, best predicted OSA status. During data analysis, researchers were blinded to which children had separately received a diagnosis of OSA. The researchers found that “The greatest AUC was achieved by the delta 12s index. At a threshold of >0.555, this identified 23/25 (sensitivity 92%) OSA cases and 89/136 true negatives (specificity 65%). The same sensitivity was achieved for 3% ODI with marginally lower specificity of 63% (86/136 true negatives).” The combined model (delta 12s index, 3% ODI, mean and minimum SpO2) detected all true positives (100% sensitivity) but with lower specificity (53%). This result would lead 60% of the sample population (12 true positives and 18 false negatives) to confirmatory multichannel studies.
The researchers concluded that "Universal screening for OSA in children with DS using simple pulse oximetry parameters could halve the number of children requiring specialist multichannel studies. Pulse oximetry is widely available, well tolerated, readily acquired in the home and its adoption could reduce the burden on health services and families alike."
The researchers noted that "Our findings specifically apply to parameters generated by Masimo oximeters and cannot be generalised to other devices. Masimo technology extracts motion artefact, this is important in children with DS who are restless sleepers." They also noted that "the use of a retrospective clinical dataset, with anonymous data shared for this analysis, limits our information on the wider sampling frame, demographic and clinical characteristics of these children."
Masimo pulse oximetry is not cleared in the United States to screen children with Down syndrome for risk of obstructive sleep apnea.
Reference
1. Hill C, Elphick H, Farquhar M, Gringras P, Pickering R, Kingshott R, Martin J, Reynolds J, Joyce A, Gavlak J, and Evans H. Home oximetry to screen for obstructive sleep apnoea in Down syndrome. Arch Dis Child. doi:10.1136/archdischild-2017-314409.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET® and Radical-7®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET® and Radical-7, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

H Michael Cohen Joins Masimo’s Board of Directors
IRVINE, Calif – August 02, 2018 – Masimo(NASDAQ: MASI), a global medical technology developer and manufacturer of innovative noninvasive patient monitoring technologies, announced today that H Michael Cohen, former Vice Chairman of Healthcare Investment Banking at Deutsche Bank Securities, has been elected to Masimo’s Board of Directors.
Mr. Cohen has almost 30 years of experience in the healthcare industry. Over the past 19 years, he has held various roles at Deutsche Bank, including Global Head, Healthcare Investment Banking and most recently Vice Chairman, Healthcare Investment Banking. Prior to joining Deutsche Bank, Mr. Cohen worked at SG Cowen, Union Bank of Switzerland, and Booz Allen Hamilton. Mr. Cohen began his career in healthcare at Hambrecht & Quist, where he was a member of the equity research team covering biotechnology, medical device, and diagnostic companies. He received his B.A. in Economics from the University of Vermont and his M.B.A. from Columbia University.
Joe Kiani, Founder, Chairman and CEO of Masimo, said, “We’re thrilled to welcome Michael to our Board of Directors. With his vast experience in healthcare investment banking and deep understanding of the medical technology industry and the complexities of global healthcare, he’ll be an excellent addition. We look forward to benefiting from his expertise and insight as Masimo continues to grow.”
“I’m honored to join Masimo’s Board,” said Mr. Cohen. “It’s been a privilege to observe Masimo grow from its humble beginning in 1989 into the global leader in noninvasive monitoring it has become today, and I’m excited to help shape the next stage of its journey while driving long-term value for Masimo and its shareholders.”
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.3-5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2018-19 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
3. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
4. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Eli Kammerman
Phone: (949) 297-7077
Email: ekammerman@masimo.com
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Reports Second Quarter 2018 Financial Results And Announces Board Authorization Of A New Stock Repurchase Program
Q2 2018 Highlights
- Total revenue, including royalty and other revenue, was $211.6 million;
- Product revenue increased 12.4% to $202.0 million, or 11.2% on a constant currency basis;
- Shipments of noninvasive technology boards and monitors were 58,700; and
- GAAP net income per diluted share of $0.79. Non-GAAP net income per diluted share increased 32.7% to $0.73.
IRVINE, Calif.--(BUSINESS WIRE)--Aug. 1, 2018-- Masimo (NASDAQ: MASI) today announced its financial results for the second quarter ended June 30, 2018.
Second Quarter 2018 Results:
Second quarter 2018 total revenue, including royalty and other revenue, was $211.6 million. Product revenues for the second quarter 2018 increased 12.4% to $202.0 million, or 11.2% on a constant currency basis.
During the second quarter of 2018, the Company shipped approximately 58,700 noninvasive technology boards and monitors.
The Company’s worldwide direct product revenue, which accounted for 85.4% of total product revenue, increased to $172.5 million in the second quarter 2018. OEM sales, which accounted for 14.6% of total product revenue, increased to $29.5 million for the second quarter 2018.
For the second quarter 2018, GAAP net income was $43.9 million or $0.79 per diluted share. Non-GAAP net income was $41.0 million, or $0.73 per diluted share.
Total cash and cash equivalents increased by $60.1 million during the quarter to $429.6 million, as of June 30, 2018.
As a result of the strong performance in the second quarter, Masimo is raising its guidance for fiscal year 2018. The Company now expects product revenues of $822 million, which reflects reported growth of 11.3% and constant currency growth of 10.8%. Masimo is also raising its GAAP EPS guidance to $3.07 and its non-GAAP EPS guidance to $2.90.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, “Our second quarter results reflect the broad-based success we are realizing for our innovative technologies and systems solutions to optimize patient care. We had a record quarter for shipments of our technology boards and monitors, at 58,700, a clear illustration of the rising demand for our high-value products. We are once again raising guidance for revenue and earnings in 2018 as we continue to grow our customer base and expand our product portfolio.”
Furthermore, today, Masimo is also announcing the Board’s authorization of a new stock repurchase program (2018 Repurchase Program), whereby the Company may purchase up to 5.0 million shares of its common stock over a period of up to three years. The 2018 Repurchase Program will be effective upon the expiration of the current stock repurchase program on September 11, 2018. The 2018 Repurchase Program may be carried out at the discretion of a committee comprised of the Company’s Chief Executive Officer and Chief Financial Officer through open market purchases, one or more Rule 10b5-1 trading plans, block trades and in privately negotiated transactions.
2018 Financial Guidance
The Company provided the following updated estimates for its full year 2018 guidance:
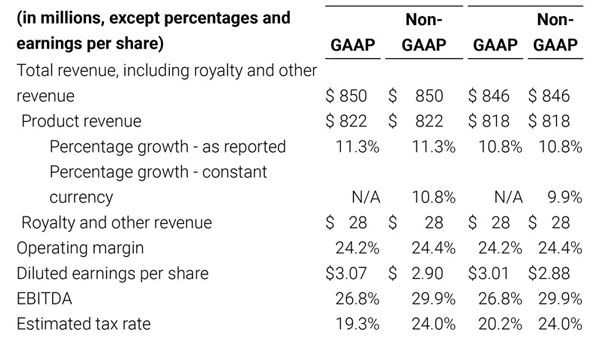
______________
1 Updated guidance provided August 1, 2018. Prior guidance provided May 2, 2018.
- Total revenue, including royalty and other revenue, increasing to $850 million;
- Product revenue increasing to $822 million; which reflects reported growth of 11.3% and constant currency growth of 10.8%;
- GAAP diluted earnings per share increasing to $3.07;
- Non-GAAP diluted earnings per share increasing to $2.90; and
- The Company expects foreign currency movements to favorably impact 2018 revenues by approximately $4 million, compared with our prior expectations of approximately $7 million.
Impact of Adoption of New Revenue Accounting Standard:
During the first quarter of 2018, the Company adopted Financial Accounting Standards Board (FASB) Accounting Standards Update No. 2014-09, Revenue (Topic 606): Revenue from Contracts with Customers (ASU 2014-09). The new revenue recognition standard requires the Company to make numerous assumptions that are based upon historical trends and management judgment. These assumptions may change over time and may have a material impact on our revenue recognition, guidance and results of operations. In accordance with the full retrospective method of adoption, the Company has adjusted certain amounts previously reported in its unaudited condensed consolidated financial statements to comply with the new standard, as indicated by the notation, “As Adjusted”. For additional information with respect to the impact of the adoption of this new accounting standard and reconciliations to the prior reported amounts, please reference Note 2 to our condensed consolidated financial statements that will be included in Part I, Item 1 of our Quarterly Report on Form 10-Q (Form 10-Q) for the quarter ended June 30, 2018 once filed with the Securities and Exchange Commission (SEC) and Exhibit 99.3 that was included in our Current Report on Form 8-K that was filed with the SEC today.
Supplementary Non-GAAP Financial Information
For additional non-GAAP financial details, please visit the Investor Relations section of the Company’s website at www.masimo.com to access Supplementary Financial Information.
Non-GAAP Financial Measures
The non-GAAP financial measures contained herein are a supplement to the corresponding financial measures prepared in accordance with U.S. GAAP. The non-GAAP financial measures presented exclude the items described below. Management believes that adjustments for these items assist investors in making comparisons of period-to-period operating results. Furthermore, management also believes that these items are not indicative of the Company’s on-going core operating performance. These non-GAAP financial measures have certain limitations in that they do not reflect all of the costs associated with the operations of the Company’s business as determined in accordance with GAAP.
Therefore, investors should consider non-GAAP financial measures in addition to, and not as a substitute for, or as superior to, measures of financial performance prepared in accordance with GAAP. The non-GAAP financial measures presented by the Company may be different from the non-GAAP financial measures used by other companies.
The Company has presented the following non-GAAP measures to assist investors in understanding the Company’s core net operating results on an on-going basis: (i) non-GAAP product revenue growth %, (ii) non-GAAP net income, (iii) non-GAAP diluted earnings per share, (iv) non-GAAP gross profit, (v) non-GAAP operating income and (vi) adjusted EBITDA. These non-GAAP financial measures may also assist investors in making comparisons of the Company’s core operating results with those of other companies. Management believes non-GAAP product revenue growth %, non-GAAP gross profit, non-GAAP operating income, non-GAAP net income, non-GAAP net income per diluted share and adjusted EBITDA are important measures in the evaluation of the Company’s performance and uses these measures to better understand and evaluate our business.
The non-GAAP financial measures reflect adjustments for the following items, as well as the related income tax effects thereof:
Constant currency foreign currency adjustments.
Some of our sales agreements with foreign customers provide for payment in currencies other than the U.S. Dollar. These foreign currency revenues, when converted into U.S. Dollars, can vary significantly from period to period depending on the average and quarter-end exchange rates during a respective period. We believe that comparing these foreign currency denominated revenues by holding the exchange rates constant with the prior year period is useful to management and investors in evaluating our product revenue growth rates on a period-to-period basis. We anticipate that fluctuations in foreign exchange rates and the related constant currency adjustments for calculation of our product revenue growth rate will continue to occur in future periods.
Acquisition-related costs, including depreciation and amortization.
Depreciation and amortization related to the revaluation of assets and liabilities (primarily intangible assets, property, plant and equipment adjustments, inventory revaluation, lease liabilities, etc.) to fair value through purchase accounting related to value created by the seller prior to the acquisition rather than ongoing costs of operating our core business. As a result, we believe that exclusion of these costs in presenting non-GAAP financial measures provides management and investors a more effective means of evaluating historical performance and projected costs and the potential for realizing cost efficiencies within our core business. Depreciation and amortization related to the revaluation of acquisition related assets and liabilities will generally recur in future periods.
Litigation damages, awards and settlements.
In connection with litigation proceedings arising in the course of our business, we have recorded expenses as a defendant in such proceedings in the form of damages, as well as gains as a plaintiff in such proceedings in the form of litigation awards and settlement proceeds; most recently in connection with our November 2016 settlement agreement with Koninklijke Philips N.V. We believe that exclusion of these expenses and gains is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these expenses and gains are generally unrelated to our core business and/or infrequent in nature.
Realized and unrealized gains or losses from foreign currency transactions.
We are exposed to foreign currency gains or losses on outstanding foreign currency denominated receivables and payables related to certain customer sales agreements, product costs and other operating expenses. As the Company does not actively hedge these currency exposures, changes in the underlying currency rates relative to the U.S. Dollar may result in realized and unrealized foreign currency gains and losses between the time these receivables and payables arise and the time that they are settled in cash. Since such realized and unrealized foreign currency gains and losses are the result of macro-economic factors and can vary significantly from one period to the next, we believe that exclusion of such realized and unrealized gains and losses are useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. Realized and unrealized foreign currency gains and losses are likely to recur in future periods.
Excess tax benefits from stock-based compensation.
Current authoritative accounting guidance requires that excess tax benefits or costs recognized on stock-based compensation expense be reflected in our provision for income taxes rather than paid-in capital. Since we cannot control or predict when stock option awards will be exercised or the price at which such awards will be exercised, the impact of such guidance can create significant volatility in our effective tax rate from one period to the next. We believe that exclusion of these excess tax benefits or costs is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. These excess tax benefits or costs will generally recur in future periods as long as we continue to issue equity awards to our employees.
Tax impacts that may not be representative of the ongoing results of our core operations.
The Tax Cuts and Jobs Act of 2017 (2017 Tax Act) was signed into law in December 2017, and became effective January 1, 2018. The 2017 Tax Act included a number of changes to existing U.S. federal tax law impacting businesses including, among other things, a permanent reduction in the corporate income tax rate from 35% to 21%, a one-time transition tax on the “deemed repatriation” of cumulative undistributed foreign earnings as of December 31, 2017 and changes in the prospective taxation of the foreign operations of U.S. multinational companies. We believe that exclusion of the tax charges related to the 2017 Tax Act is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that this tax charge is unrelated to our core business and non-recurring in nature.
Second Quarter 2018 Actuals versus Second Quarter 2017 Actuals:

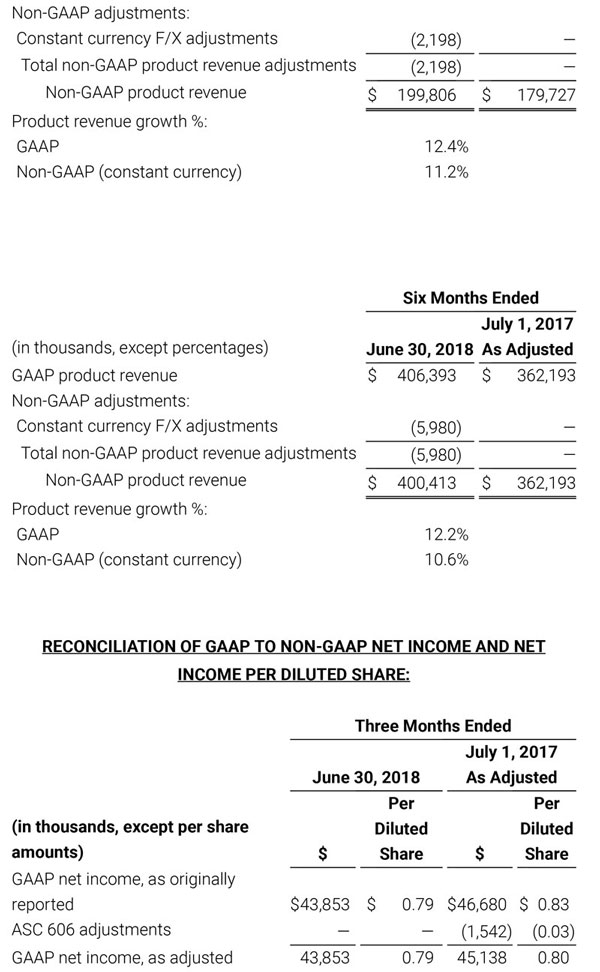
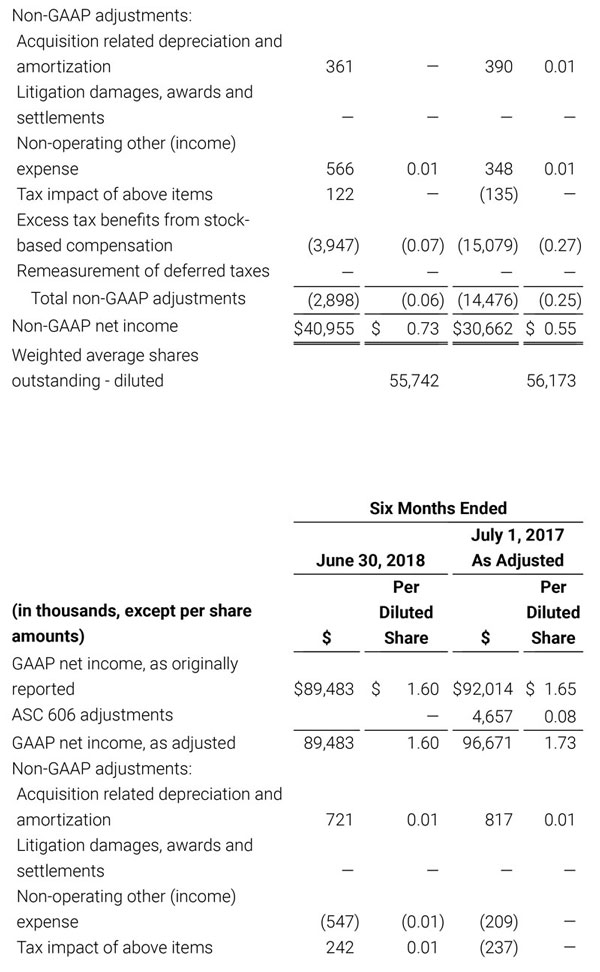
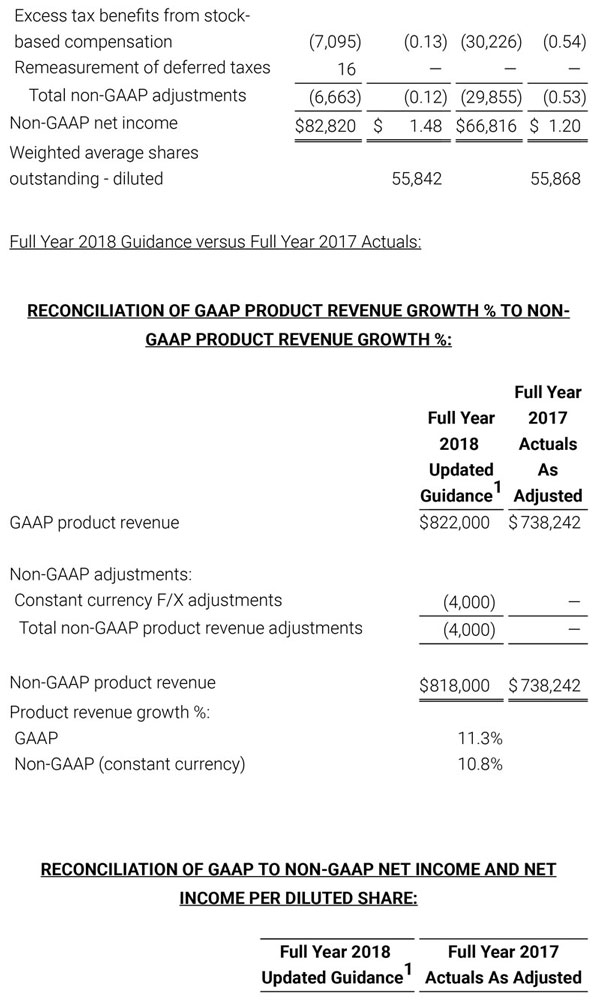
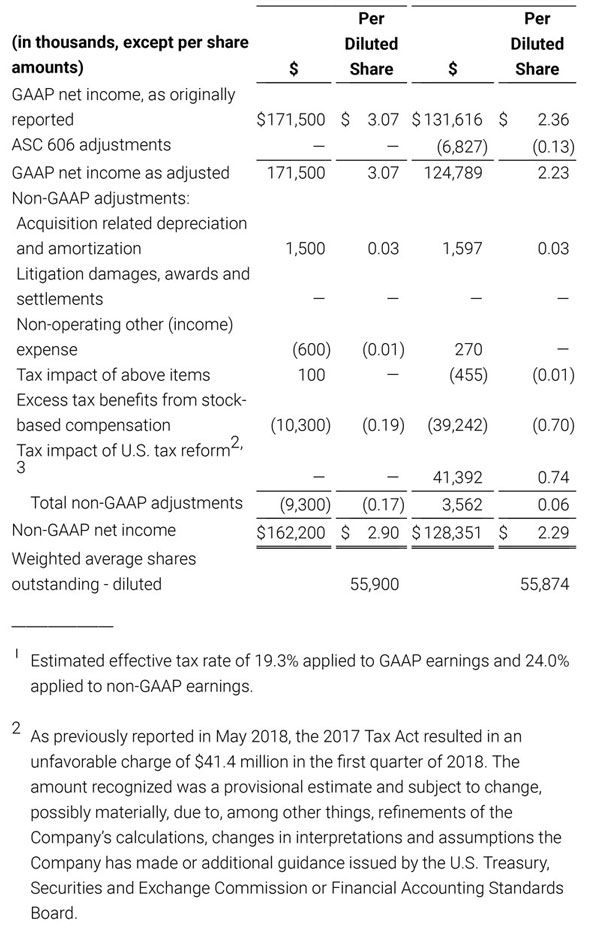
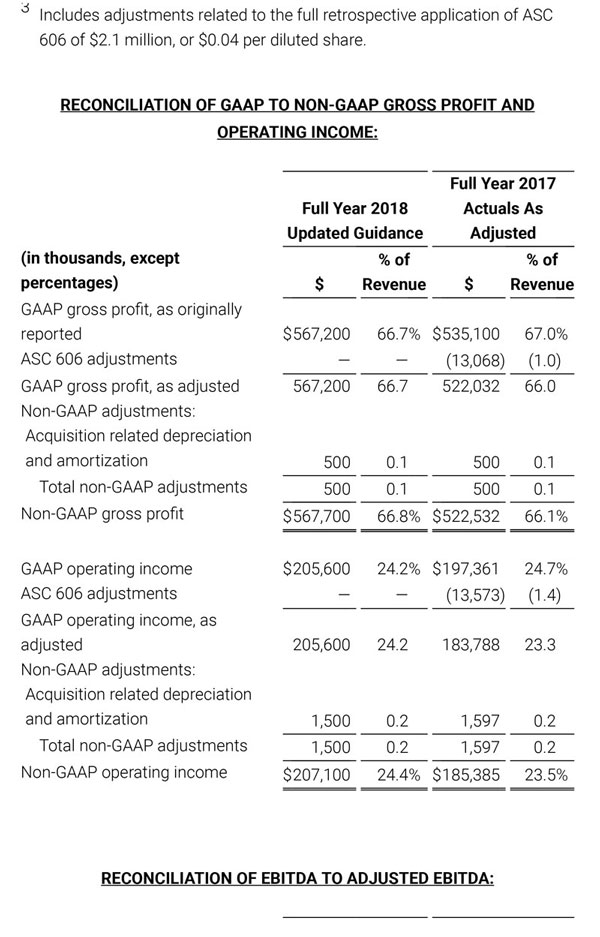
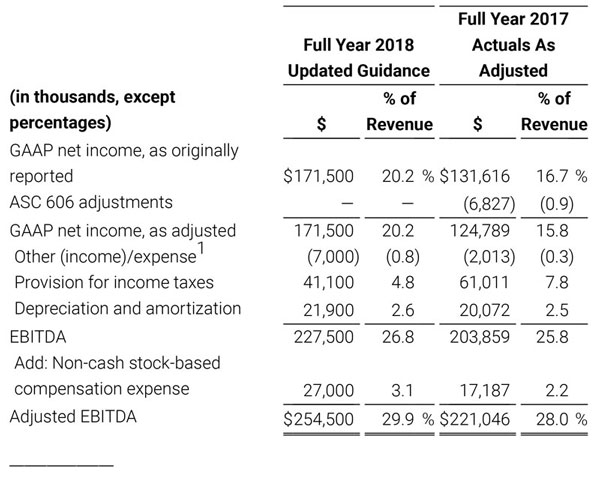
1 Other (income)/expense consists primarily of interest (income)/expense and net foreign currency (gains)/losses.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be available online from the investor relations page of the Company’s website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 7397909. After the live webcast, the call will be available on Masimo’s website through August 29, 2018. In addition, a telephonic replay of the call will be available through August 8, 2018. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 7397909.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the Company debuted Masimo SET® Measure-through Motion and Low Perfusion® pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®) and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate and perfusion index (PI). In 2014, Masimo introduced Root™, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface. Masimo is also taking an active leadership role in mobile health applications (mHealth) with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year GAAP and non-GAAP 2018 total, product, royalty and other revenues, earnings per diluted share, operating margin, EBITDA, and estimated tax rate, and our long-term outlook; demand for our products; anticipated revenue and earnings growth; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management’s current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow SET™ products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors’ assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the “Risk Factors” section of our most recent periodic reports filed with the Securities and Exchange Commission (“SEC”), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI and ORI are trademarks or registered trademarks of Masimo Corporation.
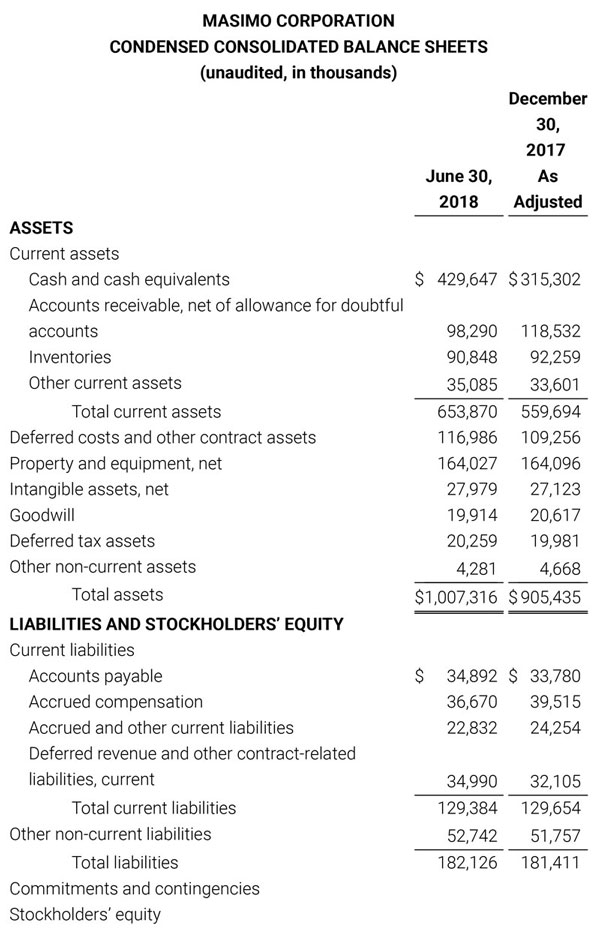
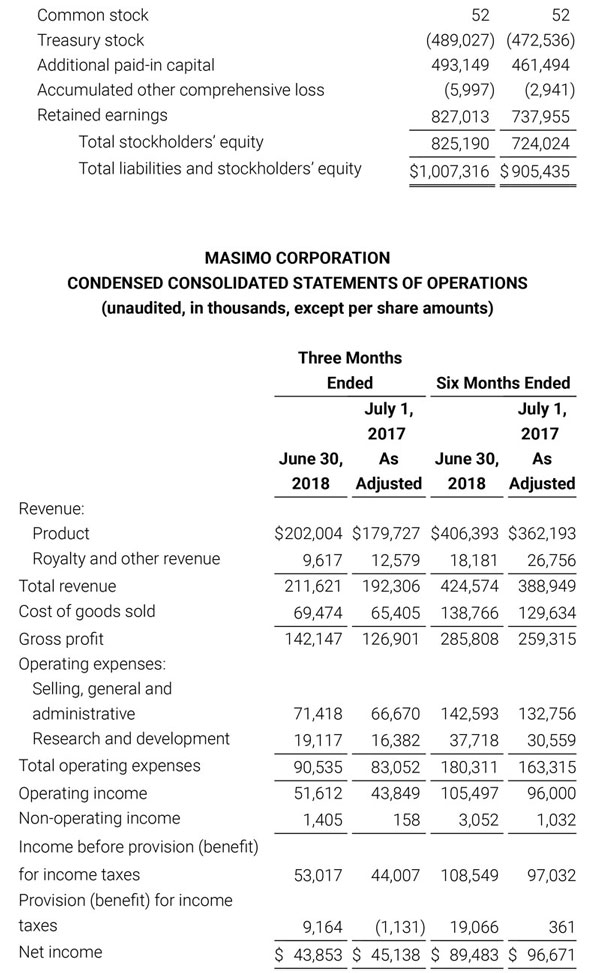
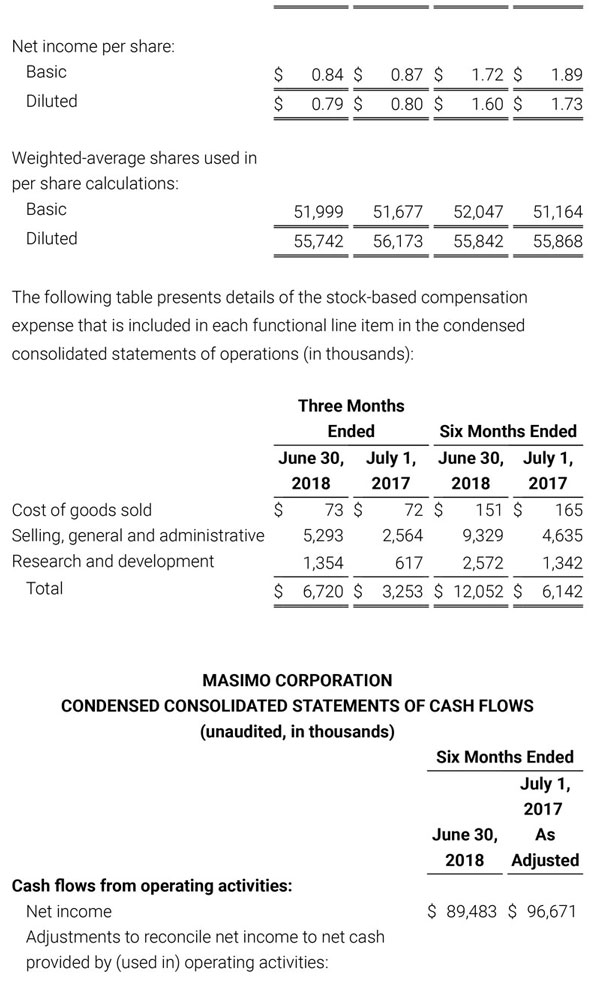
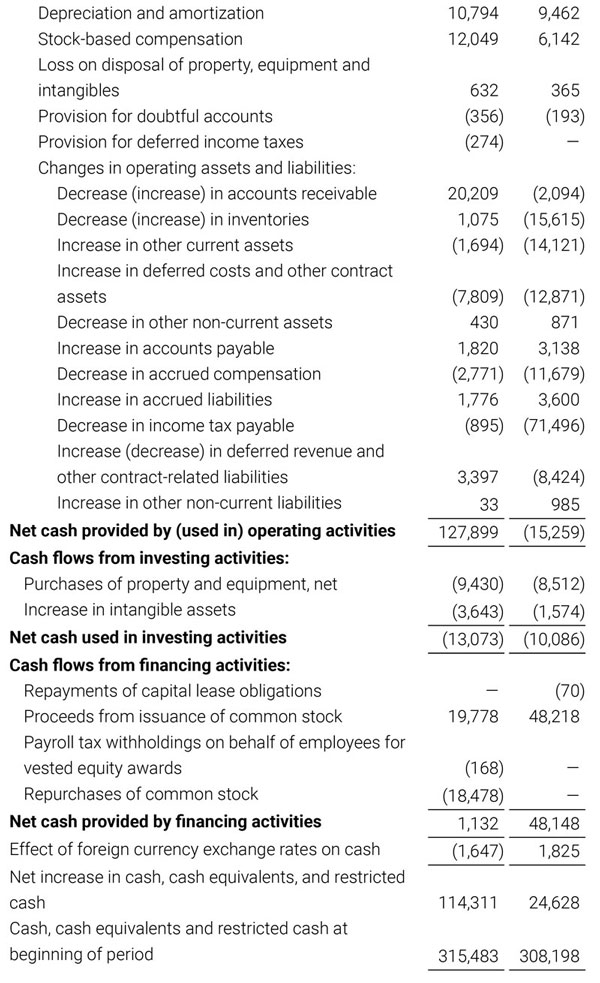

View source version on businesswire.com: https://www.businesswire.com/news/home/20180801005949/en/
Source: Masimo
Masimo
Investor Contact: Eli Kammerman
(949) 297-7077
ekammerman@masimo.com
or
Media Contact: Irene Paigah
(858) 859-7001
irenep@masimo.com

New Study Investigates Impact of General Ward Clinical Monitoring Using Masimo Root®, Radius-7®, and Patient SafetyNet™ on Clinical Workflow and Patient Care
Lebanon, New Hampshire – July 26, 2018 – Masimo (NASDAQ: MASI) announced today the findings of a recently published study in which researchers at Dartmouth-Hitchcock Medical Center investigated the impact of an integrated clinical monitoring system, using various Masimo technologies and devices, on clinical workflow and patient care in the general ward. The researchers sought to "demonstrate the application of systems-level design and analysis to measure the impact of clinical monitoring on key workflow and system characteristics that contribute to early detection of patient deterioration."1

- Masimo Radius-7®
To evaluate workflow impact through use of the enhanced monitoring system, Dr. McGrath and colleagues collected data in a study unit consisting of 2 general wards with 71 beds total for five months prior to and five months after implementation. They also collected the same data for the full ten months in a control unit consisting of 2 general wards with 61 beds total, which did not have any system changes. In both the study and control units, prior to implementation, the baseline monitoring system consisted primarily of Masimo Rad-87® Pulse CO-Oximeters®, for continuous and spot-check (vital signs) measurements using Masimo SET® pulse oximetry, and Masimo Patient SafetyNet™, a supplemental remote monitoring and clinician notification system, used for data processing and archiving.
The enhanced monitoring system, implemented in the study unit, added Masimo Root® with Radius-7® wearable Pulse CO-Oximeters. Root is a patient monitoring and connectivity platform that includes such features as built-in blood pressure and temperature measurements, a barcode reader and integration with the hospital’s admission-discharge-transfer (ADT) system, and integration with Patient SafetyNet and the hospital’s electronic medical record (EMR) system for automated capture of patient monitoring and vital signs data, including from connected third-party devices. Radius-7 is a tetherless, wearable monitor that allows patients to be mobile while still being continuously monitored, with data sent wirelessly via Bluetooth® or WiFi to Root, eliminating the need for nurses to manually place bedside monitors in standby mode and disconnect sensors each time a patient leaves the bed.
Key points of comparison and results included:
• Monitoring system utilization: The researchers noted a significant increase in the number of hours patients were continuously monitored after implementation. Monitored hours per patient day increased from mean 17.26 hours to 19.57 hours (p < 0.0001) and monitored hours per month from mean 15,931.25 hours to 19,053.3 hours (p < 0.0001).
• Vital signs documentation: With the implementation of Root and its ability to automatically upload patient data, including pulse oximetry and blood pressure and temperature measurements, to Patient SafetyNet and the EMR, researchers noted a significant decrease in the time required to obtain and record vital signs: mean assessment time dropped from 178.8 seconds to 128.9 seconds (p < 0.0001), representing an average time savings of 3 hours per day in a 36-bed unit.
• Patient information: The researchers measured the rate at which certain patient data fields were filled out in the EMR for one month before and after implementation. Patient last name presence increased from 98.92% to 100% presence (p = 0.0083). Patient first name and room and bed presence increased from 33.75% and 57.27%, respectively, to 100% (p < 0.0001).
• Clinical staff satisfaction: Three months after implementation, hospital staff feedback was solicited in a 16-question survey which had a 65% response rate and overall "very high" satisfaction with the enhanced monitoring system.
• Alarms: The researchers found that there was a significant increase in the number of clinical alarms per patient day (rate ratio 1.46, p = 0.0263) but not per monitored hour (rate ratio 1.34, p = 0.1090), which they believe is "logical when considering [the] additional time each patient [was] monitored."
The researchers concluded, "The enhanced monitoring system received high staff satisfaction ratings and significantly improved key clinical elements related to early recognition of changes in patient state, including reducing average vital signs data collection time by 28%, increasing patient monitoring time (rate ratio 1.22), and availability and accuracy of patient information. Impact on clinical alarms was mixed, with no significant increase in clinical alarms per monitored hour."
In previous studies conducted at Dartmouth-Hitchcock, researchers found that continuous monitoring of adult post-surgical patients using Masimo SET®, in conjunction with Masimo Patient SafetyNet, resulted in a 65% reduction in rapid response team activations and a 48% reduction in transfers back to the ICU.2 Over five years, they achieved their goal of zero preventable deaths or brain damage due to opioids,3 and over ten years, they maintained a 50% reduction in unplanned transfers and a 60% reduction in rescue events, despite increase in patient acuity and occupancy.4
Joe Kiani, Founder and CEO of Masimo, said, "We are incredibly grateful to Dartmouth-Hitchcock for their continued long-term research into the utility of continuous patient monitoring on the general floor and the benefits that holistic, integrated monitoring systems can provide. Continuous monitoring of all patients on opioids is clearly the path forward, with the potential to make significant improvements in patient safety and quality of care. We look forward to continuing to learn from Dartmouth-Hitchcock’s data and to improving our technologies and integrated solutions."
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. McGrath S, Perreard I, Garland M, Converse K, and Mackenzie T. Improving patient safety and clinician workflow in the general care setting with enhanced surveillance monitoring. J Biomed Health Infor. DOI 10.1109/JBHI.2018.2834863.
2. Taenzer A et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
3. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
4. McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET®, Root®, Radius-7®, and Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET®, Root, Radius-7, and Patient SafetyNet, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

New Study Investigates the Utility of Masimo PVi® as Part of Goal-Directed Fluid Management in Patients Undergoing Colorectal Surgery
IIzmit, Turkey – July 16, 2018 – Masimo (NASDAQ: MASI) announced today the findings of a recently published study in which researchers at Kocaeli University in Turkey compared the performance of conventional fluid management (CFM) to goal-directed fluid management (GDFM) using Masimo PVi® (pleth variability index, measured noninvasively and continuously using SET® pulse oximetry sensors) in patients undergoing elective colorectal surgery. The primary points of comparison were the amount of crystalloids administered and blood lactate and serum creatinine levels during the intraoperative period.1
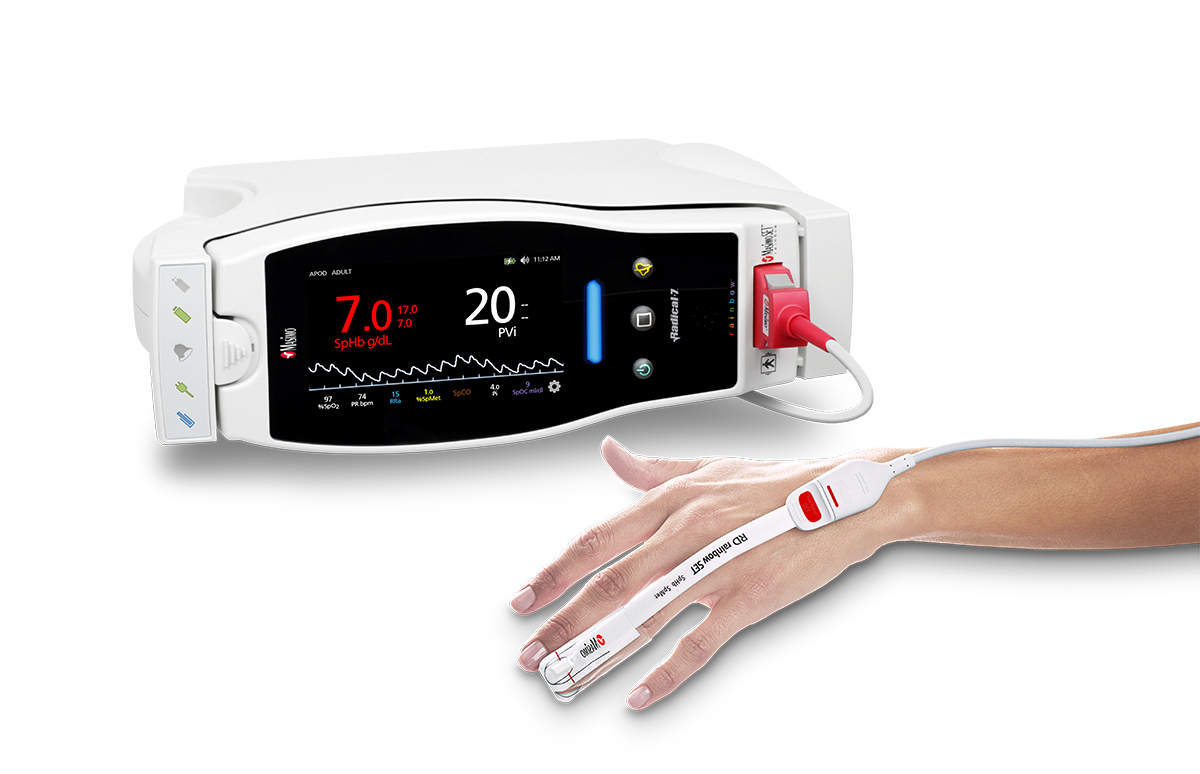
- Masimo Radical-7® with PVi®, SpHb®, and RD rainbow SET™ Sensor (Photo: Business Wire)
In the study, Dr. Cesur and colleagues, noting the importance of intraoperative fluid management in terms of postoperative organ perfusion and complications, sought to compare the effects of CFM (guided by clinical assessment and heart rate, arterial blood pressure, and invasively measured central venous pressure) with GDFM (guided by clinical assessment and noninvasive Masimo PVi monitoring). They enrolled 70 ASA I-II adult patients undergoing elective colorectal tumor surgery, who were divided randomly into CFM and GDFM groups. PVi was measured using a Masimo Radical-7® Pulse CO-Oximeter® with software version 7.0.3.3 and SET® sensors. In the CFM group, an NaCl solution was administered at the rate of 4-8 ml/kg/h; when mean arterial pressure (MAP) fell below 65 mmHg or 30% baseline MAP, the speed of infusion was increased, colloid was initiated, and ephedrine was administered. In the GDFM group, the same solution was administered at the rate of 2 ml/kg/h; if PVi rose above 13% for more than 5 minutes, colloid and then ephedrine were administered. Treatments in both groups were continued until values were restored to each protocol’s pre-treatment threshold.
The researchers found that intraoperative crystalloid administration, urine output, and end-surgery fluid balance were significantly lower in the GDFM (PVi) group:
| Characteristic | Median value (25-75 percentile values): CFM group | Median value (25-75 percentile values): GDFM (PVi) group | P-value |
|---|---|---|---|
| Intraoperative crystalloid administration | 1946 ml (1500-2500 ml) | 900 ml (800-1060 ml) | <0.001 |
| Urine output | 400 ml (250-600 ml) | 300 ml (200-400 ml) | 0.018 |
| End-surgery fluid balance | 1400 ml (960-2250 ml) | 620 ml (410-1000 ml) | <0.001 |
Durations of anesthesia and of surgery, as well as the amounts of intraoperative bleeding and administered colloid, were similar. The length of hospital stay was also found to be similar between the two groups.
The researchers noted that a limitation of the study was that they chose the number of subjects based on the needs of their primary objective, the comparison of intraoperative fluid volume between the two protocols, so they may not have evaluated enough subjects to effectively compare secondary outcomes such as length of stay: “We determined the primary goal of this study as the amount of intraoperative fluid volume and established 35 patients were needed for each group; if postoperative complications, the length of hospital stay[, were] determined as the primary goal, perhaps our numbers [in] each group [w]ould be different.”
References
1. Cesur S, Cardakozu T, Alparslan K, Turkyilmaz N, and Yavuz O. Comparison of conventional fluid management with PVI-based goal-directed fluid management in elective colorectal surgery. J Clin Mon. 14 June 2018. https://doi.org/10.1007/s10877-018-0163-y
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces Vital Signs Check Application for the Root® Patient Monitoring and Connectivity Platform
Irvine, California – July 9, 2018 – Masimo (NASDAQ: MASI) announced today the worldwide release of the Vital Signs Check Application, an integrated patient data collection and workflow application for the Masimo Root® patient monitoring and connectivity platform. Vital Signs Check, available for new and existing Root customers through a software upgrade, augments Root's versatility by helping to automate hospital vital signs testing workflows.
-
Masimo Root® Vital Signs Check
Root is a powerful, expandable platform that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions – in a single, clinician-centric hub. Root's plug-and-play expansion capabilities allow clinicians to simplify patient monitoring by bringing together advanced rainbow SET™ Pulse CO-Oximetry, brain function monitoring, regional oximetry, capnography, and vital signs measurements on an easy-to-interpret, customizable display, empowering clinicians with important information for making patient assessments.
Root Vital Signs Check allows clinicians to streamline vital signs measurement workflows and optimize patient data management through:
- • Automated patient association, with the ability to quickly associate clinicians with their measurement sessions and patients with their data using barcode scanning or a drop-down menu that pulls data from a hospital's HL7 Admit Discharge Transfer (ADT) system.
- • Centralized data collection using a single device. Root, with the use of its optional roll stand, serves as a convenient, mobile data collection point for a variety of integrated measurements, including oxygen saturation (SpO2), pulse rate, respiration rate, noninvasive hemoglobin (SpHb®), and noninvasive blood pressure and temperature (including, in the U.S., non-contact thermometry via the newly announced TIR-1™). Clinicians can also enter up to 30 additional measurements for immediate documentation and validation at the patient's bedside, configured for each care area’s protocols.
- • Early Warning Scores. Configurable early warning scores (EWS) can be calculated using up to 14 Root-measured and manually-entered contributors, as determined by hospital protocol.
- • Immediate electronic charting at the bedside, with the ability to send complete, comprehensive data to the hospital electronic medical record (EMR) with the touch of a button. If a network connection is interrupted or only available at an access point, Root can store up to 1,000 sessions and automatically push all data to the EMR once network access becomes available, saving time and minimizing the need for manual documentation. Local storage, coupled with unique patient identifiers, also allows clinicians to trend and review progress over time from the bedside, which may help to identify patterns of deterioration.
Joe Kiani, Founder and CEO of Masimo, said, "From the operating room to the emergency room, from the ICU to the med-surg unit, Root is streamlining care, simplifying access to critical data, and improving hospital workflows, all so that clinicians can stay focused on their patients. With the Vital Signs Check app, Root’s utility is further enhanced, taking an important aspect of hospital care – the repetitive, data-and-labor-intensive measurement of patient vitals – that can benefit greatly from automation and bringing it in line with the best that modern technology offers."
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

New Study Investigates the Utility of Masimo ORi™ (Oxygen Reserve Index) in Providing Early Detection of Blood Oxygenation Decrease During One-Lung Ventilation
Morioka, Japan – July 2, 2018 – Masimo (NASDAQ: MASI) announced today the findings of a study in which researchers evaluated the ability of Masimo ORi™ (Oxygen Reserve Index) to detect declining blood oxygenation prior to standard oxygen saturation (SpO2) monitoring in patients undergoing elective thoracic surgery requiring one-lung ventilation (OLV).1
-

Masimo Root® with Radical-7® and ORi™
ORi is a noninvasive, relative indicator of a patient's oxygen reserve in the moderate hyperoxic region (partial pressure of oxygen in arterial blood [PaO2] in the range of 100 to 200 mmHg). As an "index" parameter with a unit-less scale between 0 and 1, ORi can be trended and has optional alarms to notify clinicians of changes in a patient’s oxygen reserve.
In the study, Dr. Koishi and colleagues at Iwate Medical University in Morioka, Japan sought to evaluate whether ORi could serve as an early warning of desaturation in patients undergoing OLV, as such patients are often prone to hypoxemia. To this end, they assessed whether ORi decreased earlier than SpO2 during OLV, and evaluated how well ORi correlated with an invasive PaO2 measurement. ORi and SpO2 were measured every 2 seconds using a Masimo Root® with Radical-7® Pulse CO-Oximeter® and rainbow® sensors (revision L). The anesthesiologist was blinded to the ORi values. For data analysis, the researchers defined the start of the decrease in ORi as when its value fell below 0.05 less than its highest value after OLV began, and the start of the decrease in SpO2 as when it was 1% less than the maximum SpO2 value. PaO2 was calculated as part of blood gas analysis and performed every 3 minutes during OLV, resulting in 101 pairs of measurements.
The researchers found that from the start of OLV, ORi started decreasing significantly sooner than SpO2, a mean of 171 seconds vs. a mean of 372 seconds, p<0.01 (standard deviation of 102 and 231 seconds, respectively). They also found "a significant, strong correlation" between ORi and PaO2: r2=0.671, p<0.01.
The researchers concluded, "ORi decreased significantly earlier than SpO2 during OLV, suggesting that monitoring ORi might allow earlier detection of a deterioration of blood oxygenation than monitoring SpO2 alone, contributing to a reduction in the patient’s risk of exposure to complications from OLV. However, further studies in different clinical and experimental settings are needed to evaluate the superiority of the ORi over SpO2."
ORi has not received FDA 510(k) clearance and is not yet available for sale in the United States.
Reference
1. Koishi W, Kumagai M, Ogawa S, Hongo S, and Suzuki K. Monitoring the Oxygen Reserve Index can contribute to the early detection of deterioration in blood oxygenation during one-lung ventilation. Minerva Anestesiologica. 14 May 2018. DOI: 10.23736/S0375-9393.18.12622-8.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo ORi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo ORi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


United Arab Emirates Ministry of Health & Prevention Adopts National CCHD Newborn Screening Program Using Masimo Rad-97™ Pulse CO-Oximeters® with Eve™
Dubai, United Arab Emirates – June 26, 2018 – Masimo (NASDAQ: MASI) announced today that the United Arab Emirates (UAE) Ministry of Health & Prevention (MOHAP) is adopting a national screening protocol for critical congenital heart disease (CCHD) for all newborns. As part of the program launch, the MOHAP is equipping 9 hospitals across 5 emirates, serving 50% of the UAE population, with Masimo Rad-97™ Pulse CO-Oximeters® with Eve™ CCHD Newborn Screening Application. Newborns delivered at these hospitals will now be screened for CCHD using Eve – the first large-scale installation of Eve on Rad-97, which received CE marking earlier this year.
CCHD affects approximately 2.5 to 3 newborns per 1000 live births1 and requires intervention soon after birth to prevent significant morbidity or mortality; later detection in infants also increases the risk of brain damage.2 In a study of 39,821 infants, CCHD screening sensitivity increased from 63% with physical exam alone to 83% with physical exam and Masimo SET® pulse oximetry.3 In a study of 122,738 infants – the largest CCHD screening study to date – CCHD screening sensitivity increased from 77% to 93% with the combined use of Masimo SET® and clinical assessment.4
-

Masimo Rad-97™ with Eve™
Eve, also available on the Radical-7® Pulse CO-Oximeter, combines the power of Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry with an automated pre- to post-ductal synchronization algorithm designed to reduce calculation errors. In addition, Eve simplifies the CCHD screening process by providing visual instructions, animations, and a detailed, easy-to-interpret display of screening results. The ability to label results with unique patient identifiers for both mother and newborn facilitates intuitive session management and seamless electronic charting. Eve also allows clinicians to incorporate perfusion index into screening, which has been shown to increase sensitivity to the detection of CCHD in infants with pathologically low perfusion.5
Masimo worked closely with the UAE to implement the MOHAP program, including onsite training for doctors, nurses, and midwives at each hospital. H.E. Dr. Yousif Al Serkal, Assistant Undersecretary for the Hospitals Sector, emphasized the Ministry’s strategy of "providing comprehensive and innovative health services in accordance with the highest standards of excellence, professionalism, and leadership in the health sector. The goal of the 'Newborn Critical Congenital Heart Screening Program' is to ensure that all UAE newborns are screened, and all affected infants receive appropriate confirmatory testing, counseling, and treatment to prevent complications and reduce mortality." He noted that the program has a comprehensive database and e-system for the registration of all screening test results, as well as helping to control quality and track performance through periodic reporting. Dr. Kalthoom Al Balooshi, Director of the Hospitals Department, explained that "the screening initiative will be implemented in nine main hospitals, which includes screening with pulse oximetry, a CCHD screening database, integrated EMR solutions, and awareness programs for physicians, nurses, and parents."
Jon Coleman, President of Worldwide Sales, Professional Services, and Medical Affairs for Masimo, said, "We are honored that the UAE chose Masimo to help implement this vital newborn screening process for their citizens. Masimo SET® performance and accuracy have helped to usher in reliable and cost-effective CCHD screening, as shown in the multiple studies concluding that SET® pulse oximetry, combined with clinical assessment, significantly improved CCHD screening sensitivity. We believe that Masimo SET® pulse oximetry and the Eve CCHD Screening App make for a compelling combination, and hope that more institutions and governments around the world will recognize the importance of helping their youngest patients get a great start in life."
Rad-97 offers Masimo noninvasive and continuous monitoring, through Measure-through Motion and Low Perfusion SET® pulse oximetry and upgradeable rainbow® technology, in a compact, standalone monitor that incorporates advanced customizability, connectivity, and device integration capabilities. In addition to Eve, Rad-97 is also available in configurations with integrated noninvasive blood pressure measurement and integrated NomoLine™ capnography.
@MasimoInnovates || #Masimo
References
1. Hoffman JL et al. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890-1900.
2. 2011 Legislative Report; State of Maryland, Department of Health and Mental Hygiene, State Advisory Council on Hereditary and Congenital Disorders. Recommendations on Implementation of Screening for Critical Congenital Heart Disease in Newborns. Page 7.
3. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4. Zhao et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
5. de-Wahl Ganelli et al. Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction. Acta Paediatr. 2007 Oct;96(10):1455-1459.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Masimo Rad-97™, Eve™, and Radical-7®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-97, Eve, and Radical-7, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo and PositiveID Announce the U.S. Release of TIR-1™
Irvine, California and Delray Beach, Florida – June 18, 2018 - Masimo (NASDAQ: MASI) and Thermomedics, a PositiveID (OTCQB: PSID) company, announced today the debut and U.S. market release of TIR-1™, a non-contact Bluetooth®-enabled thermometer that integrates seamlessly with the Masimo Root® patient monitoring and connectivity platform.
-
Masimo TIR-1™
TIR-1 is an easy-to-use, clinical-grade infrared thermometer that provides forehead temperature measurements across all patient populations. As a non-contact device, the thermometer reduces the possibility of cross-contamination and eliminates the need to purchase additional disposables such as probe covers, reducing cost and waste. Its simple, one-button operation delivers patient temperature results in seconds, reducing the time needed to take temperature measurements compared to traditional methods. Bluetooth connectivity, available exclusively in the customized thermometer developed by Thermomedics for Masimo, enables wireless data transfer to a connected Root monitor, automating efficient integration of an important patient vital sign into bedside devices, including early warning scores, and from there to hospital EMRs via Masimo Patient SafetyNet™* or Iris Gateway™.
Masimo Root is a powerful, expandable patient monitoring and connectivity hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions – in a single, clinician-centric platform. Root’s plug-and-play expansion capabilities allow clinicians to centralize patient monitoring by bringing together advanced rainbow SET™ Pulse CO-Oximetry, brain function monitoring, regional oximetry, capnography, and vital signs measurements – now including non-contact thermometry – on an easy-to-interpret, customizable display, empowering clinicians with more information for making patient assessments.
Joe Kiani, Founder and CEO of Masimo, said, "TIR-1 is a great example of the powerful expandability and versatility of the Root platform, and of our commitment to improving patient care through streamlined and automated workflows. This thermometer makes it easy to efficiently take the temperatures of multiple patients without having to manually record data. We’re delighted to have partnered with Thermomedics to develop an additional, easy-to-use thermometry option for Root customers."
William J. Caragol, Chairman and CEO of PositiveID, said, "Our team worked diligently to develop the TIR-1, the first Bluetooth-wireless non-contact thermometer to be paired with a vital signs monitoring system. We are very proud to bring this product to market with a world-class company like Masimo and introduce non-contact thermometry to the Root platform."
@MasimoInnovates || #Masimo
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
About Thermomedics/PositiveID
About Thermomedics/PositiveID Thermomedics, Inc. designs, develops and markets medical diagnostic equipment for professional healthcare providers. Our products are specifically designed for use in a wide range of medical settings, delivering nearly instantaneous diagnostic information. Our goal was simple from the start. We wanted to build the best equipment and bring the most cutting edge technology to frontline medical professionals. Thermomedics, Inc. is a wholly owned subsidiary of PositiveID Corporation™ (OTCQB: PSID), a life sciences tools and diagnostics company. PositiveID specializes in the development of microfluidic systems in order to detect biological threats and outbreaks, whether airborne, in a healthcare setting, or at the point of need through its ExcitePCR subsidiary. For more information on PositiveID, please visit www.psidcorp.com, or connect with PositiveID on Twitter, Facebook or LinkedIn.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo MightySat™ and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo MightySat and SET®, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Allison Tomek
Thermomedics/PositiveID
Phone: (561) 805-8044
Email: atomek@psidcorp.com
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Investigates the Utility of Masimo SedLine® Patient State Index in Monitoring Anesthesia Depth of Patients with Healthy and Cirrhotic Livers
Copenhagen, Denmark – June 11, 2018 – Masimo (NASDAQ: MASI) announced today the findings of an abstract presented at Euroanaesthesia 2018 in which researchers compared Masimo Patient State Index (PSi), a processed EEG parameter provided by SedLine® brain function monitoring (in this study, first generation SedLine), to Medtronic Bispectral Index (BIS™) in monitoring the depth of anesthesia of two groups of patients: healthy volunteers undergoing hepatotomy for liver donation and cirrhotic patients undergoing liver resection. The researchers found "excellent" agreement between the two methods as well as similar sevoflurane consumption, and found that PSi 1.0 was less affected by electrocautery.1
-
Masimo Root® with SedLine® PSi and O3® Regional Oximetry
In the study, Dr. Yassen and colleagues at Menoufia University in Shibin El Kom, Egypt sought to test agreement between two brain function monitoring indices, Masimo PSi and Medtronic BIS. They monitored 60 patients in 4 sub-groups: with healthy livers undergoing hepatotomy (15 PSi, 15 BIS) and with cirrhotic livers undergoing resection (15 PSi, 15 BIS). Sensors for both technologies were simultaneously applied and anesthetists monitoring PSi were blinded to BIS results and vice versa. To measure the level of interference from electrocautery on each index, the researchers noted whether BIS and PSi values were present (displayed) or absent each time the electrocautery unit was activated.
The researchers found "An excellent degree of reliability between PSi and BIS at all measuring points." With 804 data pairs total, they calculated an overall intra-class correlation of 0.92 (95% confidence interval of 0.91-0.93, p<0.001). Using Bland-Altman analysis, they calculated overall mean bias difference of 2.19 (95% confidence interval of 1.40-2.98, p<0.0001). PSi-guided and BIS-guided sevoflurane consumption were similar: 65.67ml±31.60ml and 68.47ml±27.63ml respectively, p=0.983.
The researchers found the following rates of incidence of electrocautery interference:
| Diathermy interference? | PSi 1.0 | BIS | Test of significance |
|---|---|---|---|
| No | 96.67% | 31.67% | Z=7.4246 (p≤0.001) |
| Yes | 3.33% | 68.33% |
The researchers concluded, "Agreement between PSi and BIS during surgery is excellent among patients with healthy or cirrhotic livers. Both can be used to monitor trends of anaesthesia depth changes and equally consumed similar sevoflurane volumes. However PSi allowed for continuous monitoring without interruptions from electrocautery."
SedLine brain function monitoring helps clinicians monitor the state of the brain under anesthesia with bilateral acquisition and processing of four leads of electroencephalogram (EEG) signals. Next Generation SedLine, now available worldwide, features an enhanced PSi with less susceptibility to EMG interference and improved performance in low power EEG cases, as well as an enhanced Multitaper Density Spectral Array (DSA).
@MasimoInnovates || #Masimo
Reference
1. Yassen K, Elsheikh M, Helal S, Badawy E, and Metwally A. Patient State Index versus Bispectral Index in cirrhotic patients and non-cirrhotics undergoing hepatic resection: A controlled randomized study. Proceedings from Euroanaesthesia 2018, Copenhagen, Denmark. #1167.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine® and PSi. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine and PSi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Investigates the Utility of Continuous Noninvasive Hemoglobin Measurement with Masimo SpHb® for Reflecting Real-time Iatrogenic Hemodilution During Incremental Fluid Administration
Copenhagen, Denmark – June 4, 2018 – Masimo (NASDAQ: MASI) announced today the findings of an abstract presented at Euroanaesthesia 2018 in which researchers compared Masimo noninvasive and continuous hemoglobin (SpHb®) to intermittent and invasive lab hemoglobin (LabHb) in reflecting iatrogenic hemodilution during incremental fluid administration of patients undergoing major surgery.1
In the study, Dr. Azriel Perel, Dr. Serban Bubenek, and colleagues at the Emergency Institute for Cardiovascular Diseases in Bucharest examined the effects of incremental fluid loading on oxygen delivery and on LabHb and SpHb as markers of possible iatrogenic hemodilution, which can necessitate blood transfusions that might otherwise be avoided. 40 adult patients undergoing major gastrointestinal or vascular surgery were enrolled. Oxygen saturation (SpO2) and SpHb were continuously measured using a Masimo Radical-7® Pulse CO-Oximeter®. LabHb and partial pressure of oxygen (PaO2) were intermittently, invasively measured using an ABL800 Radiometer. Cardiac output (CO) and stroke volume (SV) were continuously, invasively measured using an Edwards Vigileo monitor. Oxygen delivery (DO2) was calculated as: CO*((Hb*1.38*SpO2)+PaO2*0.0031)). Parameter values were recorded after induction of anesthesia (T0), and 5 minutes after successive 250 ml colloid fluid challenges (FC) (T1, T2, and T3). Patients were given the second and third fluid challenges if at each stage SV increased by at least 10%.
-
Masimo Root® with Radical-7® and SpHb®
All 40 patients received the first FC, 33 received the second, and 22 received the third. The researchers found that there was "a statistically significant decrease in mean SpHb and LabHb after each FC." For patients who received all 3 FCs, they noted that "SpHb and LabHb decreased significantly and similarly after each FC." After infusion of the full 750 ml, SpHb and LabHb decreased by 1.66±0.67 g/dL and 1.7±0.7 g/dL, respectively, a decrease in Hb values which "explains the observed decrease in the DO2."
The researchers concluded, "Fluid loading as part of goal-directed therapy may cause a paradoxical decrease in DO2 due to the development of iatrogenic hemodilution. The development of iatrogenic hemodilution is reflected by a real-time decrease in the SpHb trend, which is similar to the intermittent LabHb trend."
Dr. Azriel Perel commented, "In many studies on perioperative and septic patients, the patients who received more fluids seem to have received significantly more blood transfusions. The most probable reason for that is the development of acute dilutional anemia causing the hemoglobin value to decrease below the 'transfusion threshold.' This study clearly shows that fluid administration, given as part of the conventional perioperative goal-directed strategy aimed at maximizing the cardiac output, does indeed cause a significant acute decrease of the hemoglobin concentration, leading to a paradoxical decrease in oxygen delivery. This iatrogenic decrease in hemoglobin concentration may cause physicians to administer otherwise avoidable RBC transfusions. Our study also demonstrates that continuous monitoring of hemoglobin (SpHb) through Masimo Pulse CO-Oximetry sensors may detect the development of such iatrogenic hemodilution in real time."
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
Reference
1. Bubenek S, Valeanu L, Popescu M, Cacoveanu M, Tomescu D, and Perel A. Optimization of cardiac output by incremental fluid administration is associated with iatrogenic hemodilution and a paradoxical decrease in oxygen delivery. Proceedings from Euroanaesthesia 2018, Copenhagen, Denmark.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Women's Tennis Association to Use Masimo MightySat™ Fingertip Pulse Oximeters to Advance Player Health and Performance
-
Masimo MightySat™
St. Petersburg, Florida and Irvine, California – May 21, 2018 – The Women's Tennis Association (WTA) and Masimo (NASDAQ: MASI) announced today that the WTA's Sport Sciences and Medicine (SS&M) team will immediately begin using Masimo MightySat™ as part of their mission to provide comprehensive care for athletes on the women's professional tennis circuit.
MightySat is a fingertip pulse oximeter designed for general wellness and health applications including sports, fitness, and relaxation management. MightySat provides noninvasive measurements of oxygen saturation (SpO2), pulse rate (PR), perfusion index (Pi), PVi® (studies show changes in PVi may indicate changes in hydration, breathing effort, perfusion, or other factors1-3) and RRp™ (respiration rate based on the plethysmographic waveform). MightySat features Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry. MightySat displays all noninvasive measurements in real time, and users can also display and track their data through the Masimo Personal Health app for iOS® and Android® compatible devices.
The WTA SS&M team's primary health care providers (PHCPs – licensed physical therapists and certified athletic trainers) will use MightySat at WTA tournament events to help support the general health, wellness, and fitness of elite women's tennis players. "We're fortunate to have this new technology in the training room and on the court at the WTA. This equipment will aid our PHCPs in assessing player health and allow them to better service the immediate needs of our athletes," said Kathleen Stroia, SVP SS&M and Transitions for the WTA.
-

Coco Vandeweghe
"We have been encouraged by the feedback we have received from elite athletes and trainers about how MightySat helps them improve their health and performance. For example, they use Masimo's unique accuracy to decide on how hard to train," said Joe Kiani, Founder and CEO of Masimo. "We are excited to work with the WTA to help them use Masimo's breakthrough noninvasive technologies to make a meaningful difference in women's tennis. We have long been big fans of the sport and are excited to help these peak performing athletes reach even greater heights."
MightySat is intended for general wellness and health applications. For medical applications, Masimo offers MightySat Rx, which is available to medical professionals.
-

Andrea Hlaváčková
References
1. Schooljans A et al. Pleth Variability Index Combined with Passive Leg Raising-Induced Pulse Pressure Variation to Detect Hypovolemia in Spontaneously Breathing Patients. Acta Anaesth Belg. 2010 (61), 147-150.
2. Mathews D et al. PVi Decreases Following a Fluid Bolus Administered in the PACU to Treat Hypotension. ASA 2014. A1124.
3. Perel A. Anesth Analg. 2014 Dec;119(6):1288-92.
About the WTA
The WTA is the global leader in women’s professional sport with more than 2,500 players representing nearly 100 nations competing for a record $146 million in prize money. The 2018 WTA competitive season includes 54 events and four Grand Slams in 30 countries. In 2017, the WTA was watched around the world by a total TV audience of 500 million. The 2018 WTA competitive season concludes with the BNP Paribas WTA Finals Singapore presented by SC Global from October 21-28, 2018 and the Hengqin Life WTA Elite Trophy in Zhuhai, China from October 30-November 4, 2018. Further information on the WTA can be found at ww.wtatennis.com, facebook.com/WTA and twitter.com/WTA.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo MightySat™ and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo MightySat and SET®, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Heather Bowler
WTA
Email: hbowler@wtatennis.com
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

New Study Investigates the Economic Impact and Utility of Continuous Noninvasive Hemoglobin Measurement with Masimo SpHb® in Reducing Unnecessary Blood Transfusions
Madrid, Spain – May 14, 2018 – Masimo (NASDAQ: MASI) announced today the findings of a recently published study in which researchers evaluated the utility of Masimo noninvasive and continuous hemoglobin (SpHb®) in reducing the number of unnecessary blood transfusions, thus reducing costs and improving quality of care.1
In the study, Dr. Ribed-Sánchez and colleagues at HM Hospitales, ETSII-Universidad Nacional de Educaíon a Distancia (UNED), and CEU San Pablo University in Spain compared the transfusion outcomes of two groups of patients undergoing hip trauma surgery: those whose hemoglobin levels were measured using traditional invasive blood draws and those whose levels were monitored continuously using Masimo SpHb. The researchers’ goal was to determine whether there was a difference in the percentage of patients transfused and the number of units transfused per patient between the two groups. 115 control group patients had hemoglobin levels intermittently checked through invasive blood draws and laboratory analysis. 122 experimental group patients had hemoglobin levels monitored continuously with Masimo Radical-7® Pulse CO-Oximeters® with SpHb.
The researchers found a decrease in the percentage of patients receiving a transfusion from 48.7% in the control group to 45.1% in the experimental (SpHb) group, a 7.4% reduction. The number of units transfused per patient dropped from 1.322 to 1.156, a 12.56% decrease. Based on the average cost of a unit of blood in Spain and taking sensor cost into account, they calculated that on an annual and national level, the reduction could lead to an estimated savings of 1.756 million euros and 13,500 fewer units of blood transfused.
The researchers concluded, "Constant monitoring of the value of hemoglobin during surgeries with significant blood loss significantly reduces blood transfusions. Based on the reduction of transfusions by using this measurement technology, health facilities can significantly reduce their costs while improving quality of care."
Joe Kiani, Founder and CEO of Masimo, commented, "This is the fifth SpHb study showing a reduction in blood transfusion,1-5 which is not only expensive, but associated with mortality. In fact, all six of the clinical outcome studies on SpHb that we are aware of to date have been positive.6 In addition to blood transfusion reduction, three of the studies showed faster time to transfusion for patients who needed it."3,5,7
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Ribed-Sánchez B, González-Gaya C, Varea-Díaz S, Corbacho-Fabregat C, Pérez-Otoyza J, and Belda-Iniesta C. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
2. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedia Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
3. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
4. Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
5. Kamal A et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice during Abdominal Cancer Surgery. Open Journal of Anesthesiology, 6, 13-19. DOI: 10.4236/ojanes.2016.63003
6. Published clinical studies and abstracts on Masimo SpHb can be found at http://www.masimo.com/evidence/pulse-co-oximetry/sphb/.
7. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo SET® Pulse Oximetry Helps Form Basis of Utah Senate Resolution on Postoperative Oxygen Saturation Home Monitoring for Patients Prescribed Opioids
Salt Lake City, Utah – May 7, 2018 – The Utah State Legislature and Governor Gary Herbert have recently signed into law the Concurrent Resolution on Deaths From Opioid-Induced Postoperative Respiratory Depression (SCR4). The resolution urges hospitals, clinicians, and researchers to examine and identify possible links between opioids and respiratory depression following surgery and encourages doctors to prescribe home monitoring for applicable patients, which may alert caregivers to low oxygen saturation (SpO2) and changes in breathing which can precede cardiac arrest and death. Pulse oximetry technology was used in the case studies that helped shape the legislation, providing lawmakers with compelling evidence of the need for postoperative home SpO2 monitoring of patients who are prescribed opiate painkillers.
SCR4 is commonly referred to as "Parker's Bill" in memory of Parker Stewart, a 21-year-old newlywed who tragically passed away in his sleep three days after tonsillectomy surgery, as a result of respiratory depression believed to be caused by opioid painkillers, of which he had taken only half the prescribed dose.
Researchers at Uintah Basin Medical Center and Intermountain Healthcare – Dr. Michael Catten, Shay Uresk, BSRT, RRT, SDS, and Kim Bennion, MsHS, RRT, CHC – have been investigating the utility of home pulse oximeter use on postoperative patients. Several case studies – using Masimo (NASDAQ: MASI) SET® Measure-through Motion and Low Perfusion™ pulse oximetry and rainbow Acoustic Monitoring® with RRa® – helped illustrate the benefits of postoperative monitoring for respiratory depression. Their work formed the basis for what became "Parker's Bill," SCR4.
"Even at normal doses, opioids can cause respiratory depression and death, and yet, the technology to monitor patients who are taking opioids is minimally expensive," commented Dr. Catten. "Home monitoring can save thousands – not a few, thousands – of lives a year. SCR4 passed unanimously through all committees and houses of the Utah legislature, without dissent, and I hope we’ll be able to get other states onboard with similar home monitoring resolutions."
Moving forward and in accordance with the recommendations made in SCR4, clinicians at Uintah Basin Medical Center and Intermountain Healthcare are now prescribing home SpO2 monitoring using Masimo technology and devices, such as Rad-97™ Pulse CO-Oximeters®. Taking advantage of Rad-97's advanced connectivity capabilities when used together with Masimo Patient SafetyNet™*, a remote supplemental patient surveillance and clinician notification system, doctors are able to remotely monitor their at-home patients for signs of respiratory depression in real time, as an added precaution.
"This technology is a game changer," notes Dr. Catten. "The ability to observe and communicate with patients from afar is key. It’s like having your own EMT by your side at home, watching to make sure you’re okay after surgery. I’m so grateful to the engineers who work so hard to develop this technology, which has the potential to save so many lives."
In a landmark study at Dartmouth-Hitchcock Medical Center in New Hampshire, researchers found that continuous monitoring of adult post-surgical patients using Masimo SET® on Masimo bedside devices, in conjunction with Masimo Patient SafetyNet, resulted in a 65% reduction in rapid response team activations and a 48% reduction in transfers back to the ICU.1 Over five years, they achieved their goal of zero preventable deaths or brain damage due to opioids,2 and over ten years, they maintained a 50% reduction in unplanned transfers and a 60% reduction in rescue events, despite increase in patient acuity and occupancy.3
Joe Kiani, Founder and CEO of Masimo, commented, "We are so happy to see Utah lawmakers recognize the suffering and death that unmonitored postoperative use of prescription painkillers can lead to – and the lives that continuous SET® pulse oximetry monitoring can help to save. Masimo is honored to have been part of the research and the solution surrounding passage of this historic legislation. We hope other states and countries will follow in addressing this important patient safety issue."
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
2. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
3. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET®, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Reports First Quarter 2018 Financial Results
- • Total revenue, including royalty and other revenue was $213.0 million
- • Product revenue was $204.4 million
- • Shipments of noninvasive technology boards and monitors were 53,600
- • GAAP net income of $45.6 million, or $0.82 per diluted share. Non-GAAP net income of $41.9 million, or $0.75 per diluted share.
Irvine, California, May 2, 2018 Masimo (NASDAQ: MASI) today announced its financial results for the first quarter ended March 31, 2018.
First Quarter 2018 Results:First quarter 2018 total revenue, including royalty and other revenue, increased to $213.0 million. Product revenues for the first quarter of 2018 increased to $204.4 million.
The Company’s worldwide direct product revenue, which accounted for 87.5% of total product revenue, increased to $178.9 million in the first quarter of 2018. OEM sales, which accounted for 12.5% of total product revenue, increased to $25.5 million in the first quarter of 2018.
GAAP net income for the first quarter of 2018 was $45.6 million, or $0.82 per diluted share.
Non-GAAP net income for the first quarter of 2018 was $41.9 million, or $0.75 per diluted share.
As a result of the strong performance in the first quarter, Masimo is raising its fiscal year 2018 product revenue guidance from $808 million to $818 million, its GAAP EPS guidance from $2.90 to $3.01 and its non-GAAP EPS guidance from $2.80 to $2.88.
During the first quarter of 2018, the Company shipped approximately 53,600 noninvasive technology boards and monitors.
As of March 31, 2018, total cash and cash equivalents were $369.5 million. During the first quarter of 2018, the Company repurchased approximately 0.2 million shares of common stock at a total cost of $16.5 million.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, “We had another record quarter for product revenues and earnings, demonstrating once again the clinical and economic value of our innovative technologies for improving patient care. We achieved another strong quarter of shipments of our noninvasive monitoring technologies, at 53,600. Our positive outlook is visible in our higher guidance for sales and earnings in 2018 as we introduce important new products and gain new customers around the world.”
2018 Financial GuidanceThe Company provided the following updated estimates for its full year 2018 guidance:
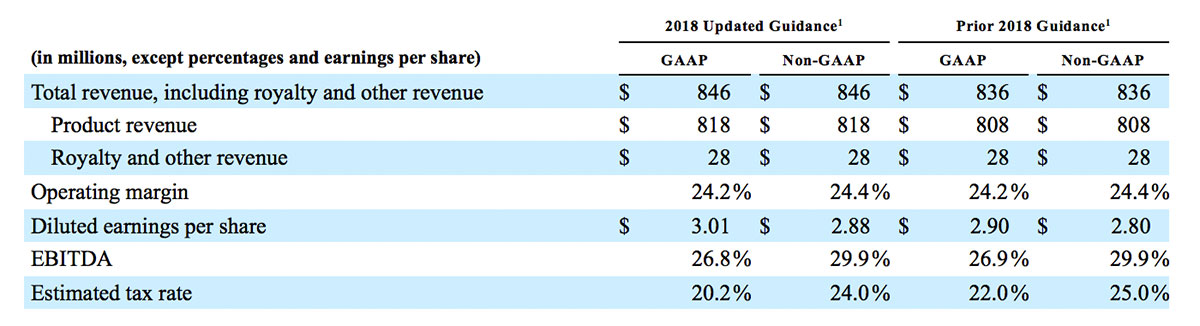
- 1. Updated guidance provided May 2, 2018. Prior guidance provided February 27, 2018.
- • Total revenue, including royalty and other revenue, increasing to $846 million;
- • Product revenue increasing to $818 million;
- • GAAP diluted earnings per share increasing to $3.01; and
- • Non-GAAP diluted earnings per share increasing to $2.88
Impact of Adoption of New Revenue Accounting Standard:
During the first quarter of 2018, the Company adopted Financial Accounting Standards Board (FASB) Accounting Standards Update No. 2014-09, Revenue (Topic 606): Revenue from Contracts with Customers (ASU 2014-09). The new revenue recognition standard requires the Company to make numerous assumptions that are based upon historical trends and management judgment. These assumptions may change over time and may have a material impact on our revenue recognition, guidance and results of operations. In accordance with the full retrospective method of adoption, the Company has adjusted certain amounts previously reported in its unaudited condensed consolidated financial statements to comply with the new standard, as indicated by the notation, “As Adjusted”. For additional information with respect to the impact of the adoption of this new accounting standard and reconciliations to the prior reported amounts, please reference Note 2 to our condensed consolidated financial statements that will be included in Part I, Item 1 of our Quarterly Report on Form 10-Q (Form 10-Q) for the quarter ended March 31, 2018 once filed with the Securities and Exchange Commission (SEC) and Exhibit 99.3 that was included in our Current Report on Form 8-K that was filed with the SEC today.
Supplementary Non-GAAP Financial InformationFor additional non-GAAP financial details, please visit the Investor Relations section of the Company’s website at www.masimo.com to access Supplementary Financial Information.
Non-GAAP Financial Measures
The non-GAAP financial measures contained herein are a supplement to the corresponding financial measures prepared in accordance with U.S. GAAP. The non-GAAP financial measures presented exclude the items described below. Management believes that adjustments for these items assist investors in making comparisons of period-to-period operating results. Furthermore, management also believes that these items are not indicative of the Company’s on-going core operating performance. These non-GAAP financial measures have certain limitations in that they do not reflect all of the costs associated with the operations of the Company’s business as determined in accordance with GAAP.
Therefore, investors should consider non-GAAP financial measures in addition to, and not as a substitute for, or as superior to, measures of financial performance prepared in accordance with GAAP. The non-GAAP financial measures presented by the Company may be different from the non-GAAP financial measures used by other companies.
The Company has presented the following non-GAAP measures to assist investors in understanding the Company’s core net operating results on an on-going basis: (i) non-GAAP net income, (ii) non-GAAP diluted earnings per share, (iii) non-GAAP gross profit, (iv) non-GAAP operating income and (v) adjusted EBITDA. These non-GAAP financial measures may also assist investors in making comparisons of the Company’s core operating results with those of other companies. Management believes non-GAAP gross profit, non-GAAP operating income, non-GAAP net income, non-GAAP net income per diluted share and adjusted EBITDA are important measures in the evaluation of the Company’s performance and uses these measures to better understand and evaluate our business.
The non-GAAP financial measures reflect adjustments for the following items, as well as the related income tax effects thereof:
Acquisition-related costs, including depreciation and amortization.
Depreciation and amortization related to the revaluation of assets and liabilities (primarily intangible assets, property, plant and equipment adjustments, inventory revaluation, lease liabilities, etc.) to fair value through purchase accounting related to value created by the seller prior to the acquisition rather than ongoing costs of operating our core business. As a result, we believe that exclusion of these costs in presenting non-GAAP financial measures provides management and investors a more effective means of evaluating historical performance and projected costs and the potential for realizing cost efficiencies within our core business. Depreciation and amortization related to the revaluation of acquisition related assets and liabilities will generally recur in future periods.
Litigation damages, awards and settlements.
In connection with litigation proceedings arising in the course of our business, we have recorded expenses as a defendant in such proceedings in the form of damages, as well as gains as a plaintiff in such proceedings in the form of litigation awards and settlement proceeds; most recently in connection with our November 2016 settlement agreement with Koninklijke Philips N.V. We believe that exclusion of these expenses and gains is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these expenses and gains are generally unrelated to our core business and/or infrequent in nature.
Realized and unrealized gains or losses from foreign currency transactions.
We are exposed to foreign currency gains or losses on outstanding foreign currency denominated receivables and payables related to certain customer sales agreements, product costs and other operating expenses. As the Company does not actively hedge these currency exposures, changes in the underlying currency rates relative to the U.S. Dollar may result in realized and unrealized foreign currency gains and losses between the time these receivables and payables arise and the time that they are settled in cash. Since such realized and unrealized foreign currency gains and losses are the result of macro-economic factors and can vary significantly from one period to the next, we believe that exclusion of such realized and unrealized gains and losses are useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. Realized and unrealized foreign currency gains and losses are likely to recur in future periods.
Excess tax benefits from stock-based compensation.
Current authoritative accounting guidance requires that excess tax benefits or costs recognized on stock-based compensation expense be reflected in our provision for income taxes rather than paid-in capital. Since we cannot control or predict when stock option awards will be exercised or the price at which such awards will be exercised, the impact of such guidance can create significant volatility in our effective tax rate from one period to the next. We believe that exclusion of these excess tax benefits or costs is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. These excess tax benefits or costs will generally recur in future periods as long as we continue to issue equity awards to our employees.
Tax impacts that may not be representative of the ongoing results of our core operations.
The Tax Cuts and Jobs Act of 2017 (2017 Tax Act) was signed into law in December 2017, and became effective January 1, 2018. The 2017 Tax Act included a number of changes to existing U.S. federal tax law impacting businesses including, among other things, a permanent reduction in the corporate income tax rate from 35% to 21%, a one-time transition tax on the “deemed repatriation” of cumulative undistributed foreign earnings as of December 31, 2017 and changes in the prospective taxation of the foreign operations of U.S. multinational companies. We believe that exclusion of the tax charges related to the 2017 Tax Act is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that this tax charge is unrelated to our core business and non-recurring in nature.
First Quarter 2018 Actuals versus First Quarter 2017 Actuals:
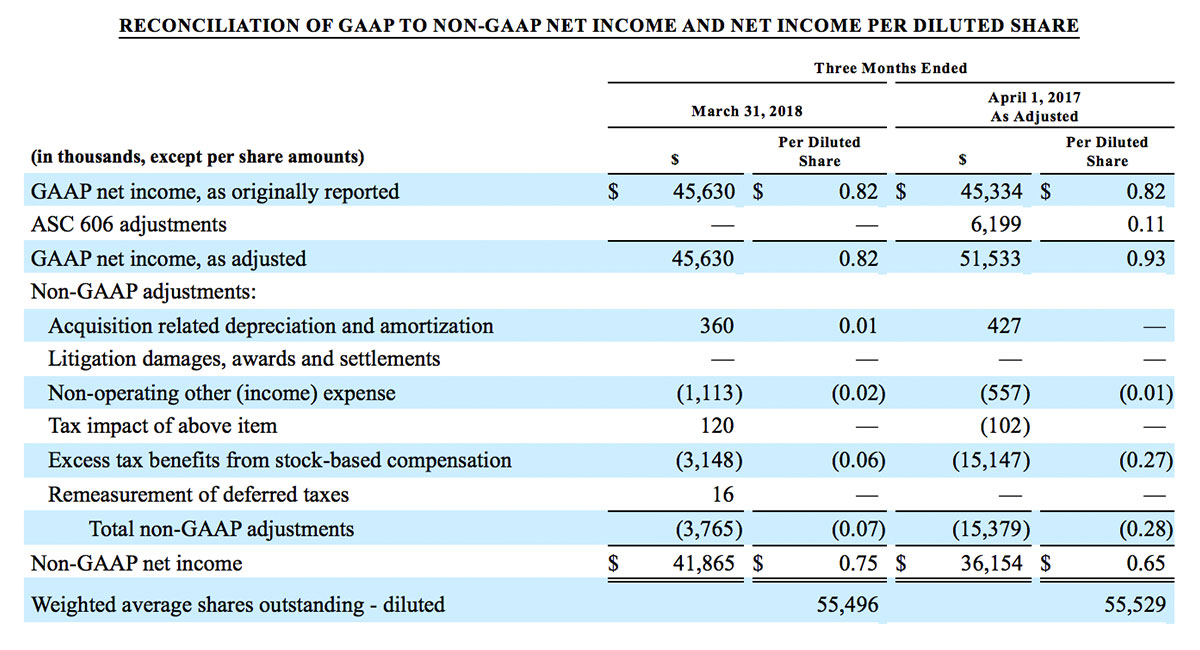
Full Year 2017 Actuals versus Full Year 2018 Guidance:
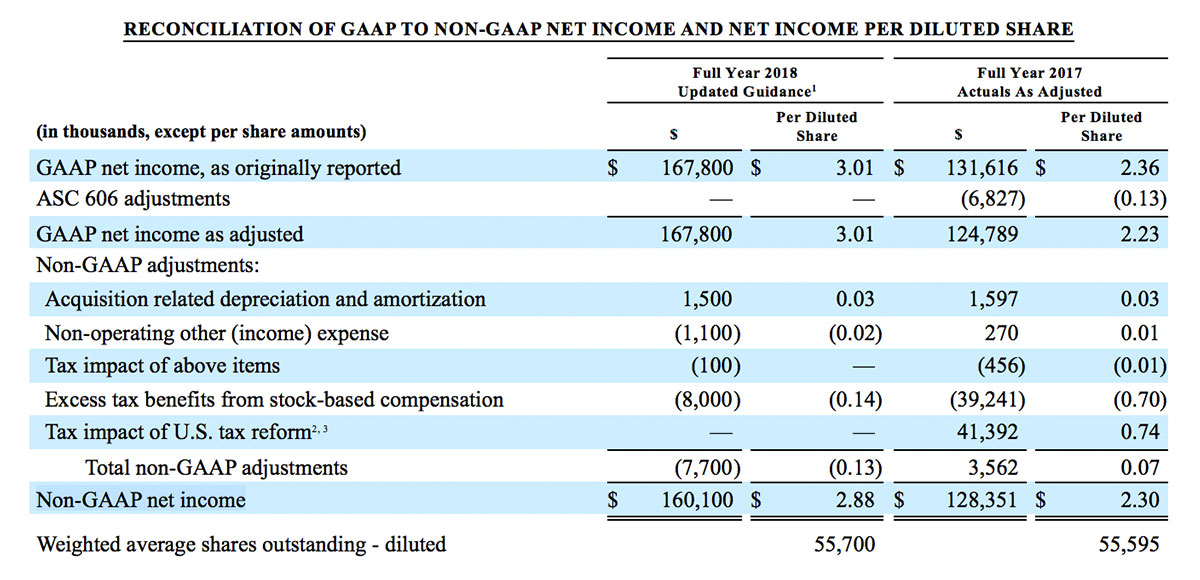
- 1. Estimated effective tax rate of 22% applied to GAAP earnings and 25% applied to non-GAAP earnings.
- 2. As previously reported in February 2018, the 2017 Tax Act resulted in an unfavorable charge of $43.5 million in the fourth quarter of 2017. The amount recognized was a provisional estimate and subject to change, possibly materially, due to, among other things, refinements of the Company’s calculations, changes in interpretations and assumptions the Company has made or additional guidance issued by the U.S. Treasury, Securities and Exchange Commission or Financial Accounting Standards Board.
- 3. Includes adjustments related to the full retrospective application of ASC 606 of $2.1 million, or $0.04 per diluted share.
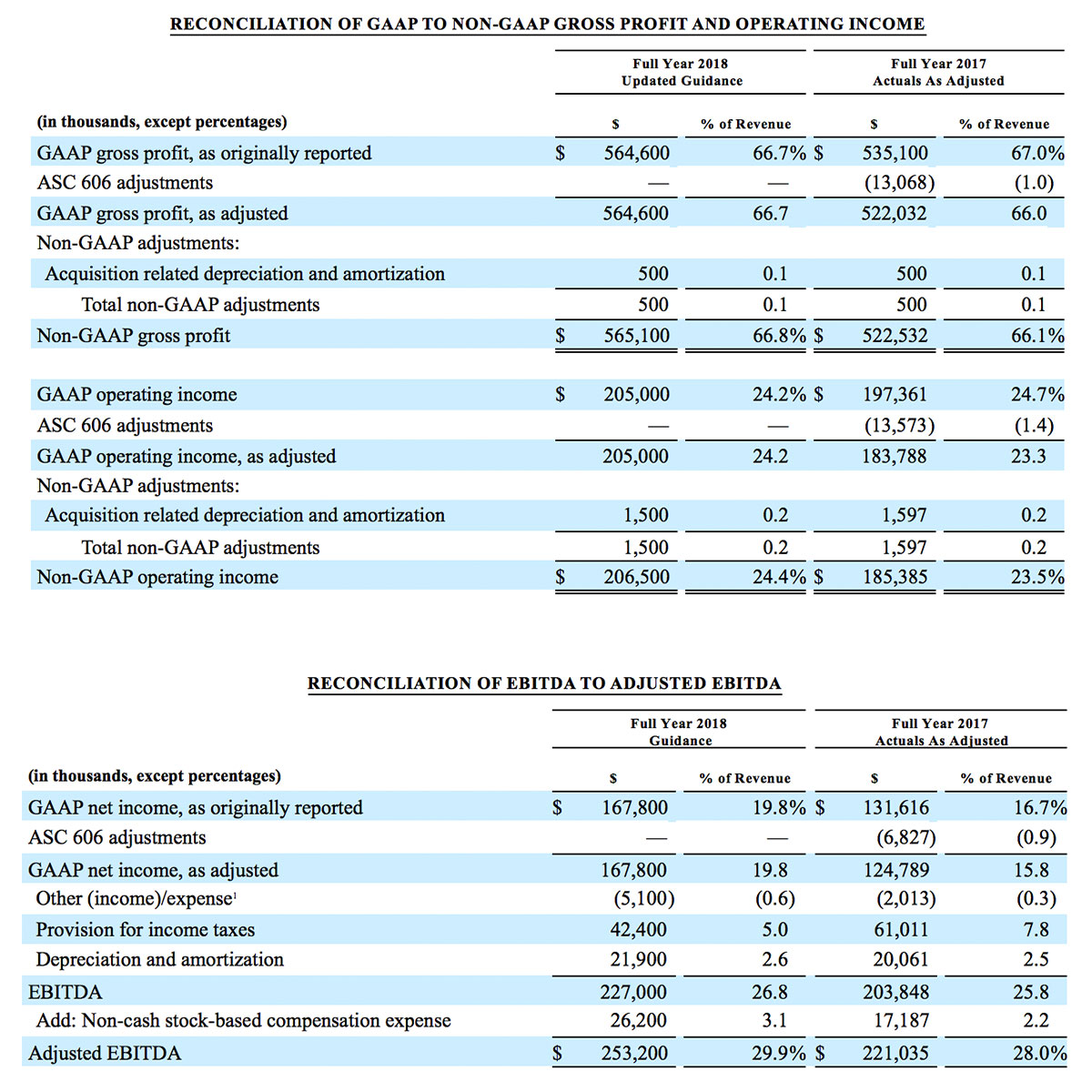
- 1. Other (income)/expense consists primarily of interest (income)/expense and net foreign currency (gains)/losses.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be available online from the investor relations page of the Company’s website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 4776719. After the live webcast, the call will be available on Masimo’s website through May 30, 2018. In addition, a telephonic replay of the call will be available through May 9, 2018. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 4776719.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the Company debuted Masimo SET® Measure-through Motion and Low Perfusion® pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®) and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate and perfusion index (PI). In 2014, Masimo introduced Root™, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface. Masimo is also taking an active leadership role in mobile health applications (mHealth) with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at http://www2.masimo.com/evidence/featured-studies/feature/.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year GAAP and non-GAAP 2018 total, product, royalty and other revenues, earnings per diluted share, operating margin, EBITDA, and estimated tax rate, and our long-term outlook; demand for our products; anticipated revenue and earnings growth; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management’s current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow SET™ products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors’ assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the “Risk Factors” section of our most recent periodic reports filed with the Securities and Exchange Commission (“SEC”), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact: Eli Kammerman
Phone: (949) 297-7077
Email: ekammerman@masimo.com
Media Contact: Irene Paigah
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI and ORI are trademarks or registered trademarks of Masimo Corporation.
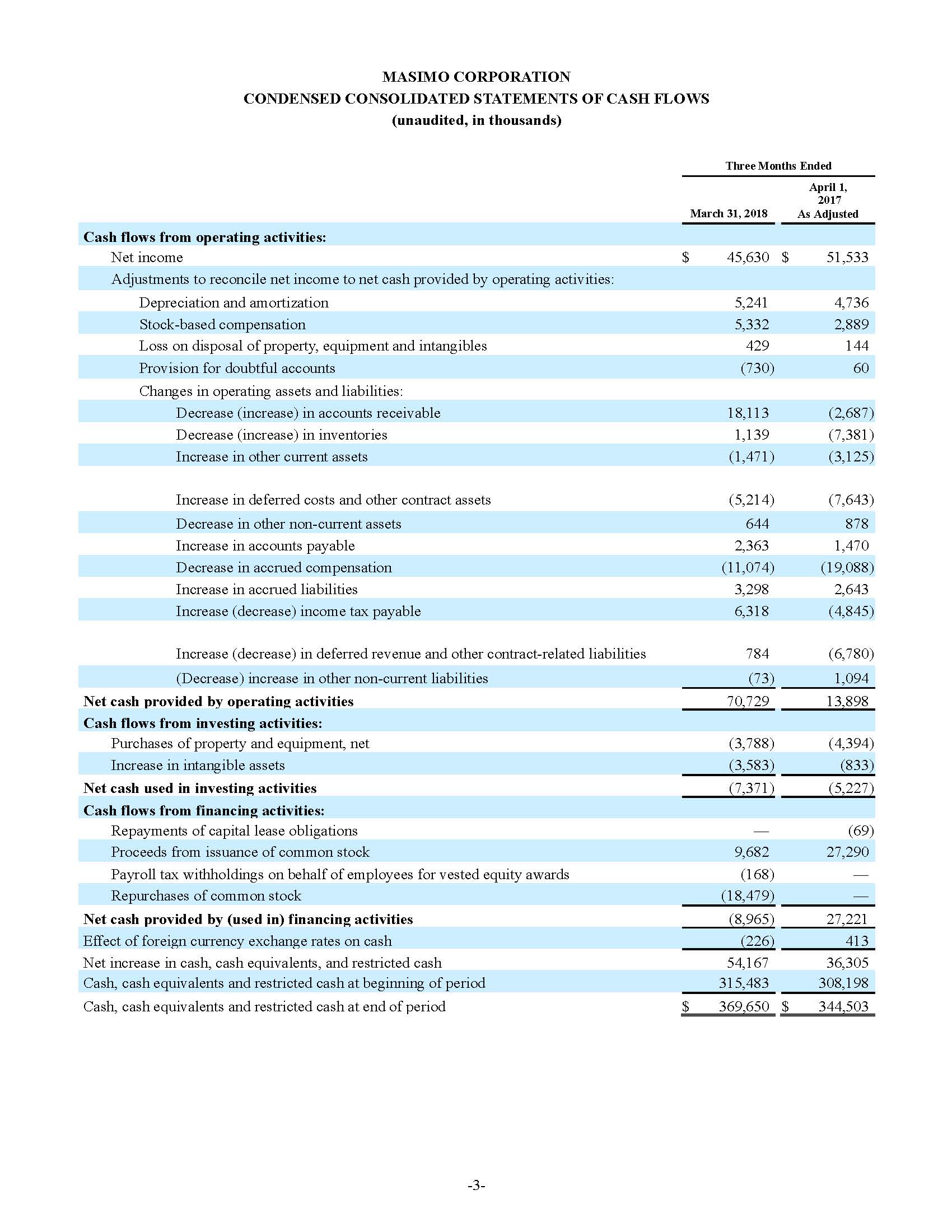

Masimo O3® Regional Oximetry and SedLine® Brain Function Monitoring Power Two Investigations into Postoperative Delirium Presented at IARS
Chicago, Illinois – May 1, 2018 – Masimo (NASDAQ: MASI) announced today the use of Masimo technologies – O3® regional oximetry and SedLine® brain function monitoring – in two abstracts presented at the 2018 Annual Meeting of the International Anesthesia Research Society (IARS). Both studies investigated links between postoperative delirium and particular biomarkers.
-
Masimo Root® Patient Monitoring and Connectivity Platform with O3® Regional Oximetry and SedLine® Brain Function Monitoring
In the first study1, Dr. Aymen Benkreira and colleagues at the Montreal Heart Institute sought to identify a possible link between venous congestion from right ventricular failure and postoperative delirium after cardiac surgery. As a proxy for venous congestion, they investigated whether there was a link between portal vein pulsatility (evaluated by point-of-care Doppler ultrasound) and postoperative delirium and associated features. In addition to daily cognitive and delirium evaluations for five consecutive days, the patients were evaluated for asterixis and bilateral cerebral oximetry values using Masimo O3. Of the 145 adult patients enrolled in the study, an abnormal cognitive status was identified in 36.9% of assessments and portal vein pulsatility in 42.3% of assessments. The researchers found significant associations between portal pulsatility and:
- • the development of cognitive dysfunction (odds ratio of 2.10, confidence interval of 1.25-3.53, p=0.005)
- • the development of asterixis (OR of 2.23, CI of 1.13-4.41, p=0.02)
- • a more than 15% decrease in cerebral oximetry values from pre-operative baseline (OR of 2.23, CI of 1.12-4.71, p=0.02).
The researchers concluded, "This data presents for the first time an association between an objective sign of portal hypertension of cardiac origin and delirium in cardiac surgery patients. This suggests that delirium may have a congestive origin in some patients."
In the second study2, Reine Ibala and colleagues at Massachusetts General Hospital sought to identify EEG biomarkers that could aid clinicians in preemptively identifying patients at risk for postoperative delirium. Such biomarkers – particular alpha and beta band oscillatory dynamics that may reflect the robustness of cortical circuits underlying cognitive processes – are commonly present in patients with clinically diagnosed cognitive impairment (dementia). The researchers theorized that there might be a link between dementia and postoperative delirium and thus also between postoperative delirium and cognitively normal patients whose EEG data exhibit similar biomarkers under anesthesia. They analyzed EEG data, collected using Masimo SedLine brain function monitoring, from 24 patients (21 cognitively normal, 3 with dementia). Analysis demonstrated a significant association between EEG point estimates for alpha/beta power and dementia (odds ratio of 1.43, 95% confidence interval of 1.14 to 1.79). Postoperative delirium for cognitively normal patients was associated with "visually evident decreased alpha/beta power," passing the threshold of statistical significance.
The researchers concluded, "Decreased alpha/beta oscillation power during sevoflurane general anesthesia is strongly associated with known cognitive impairment. This dynamic may be used to develop principled neurophysiological-based approaches to aid the preemptive identification and care targeting of vulnerable patients. Cognitively normal patients who developed postoperative delirium demonstrated a similar neurophysiological profile – but to a lesser extent – compared to patients with dementia to suggest that postoperative delirium may be more closely associated with sub-clinical cognitive impairment than previously appreciated."
SedLine brain function monitoring helps clinicians monitor the state of the brain under anesthesia with bilateral acquisition and processing of four leads of electroencephalogram (EEG) signals. Next Generation SedLine, recently released, features an enhanced signal processing engine, driving a variety of performance improvements, and an enhanced Multitaper Density Spectral Array (DSA). Next Generation SedLine can be used simultaneously with O3 regional oximetry on the Root® patient monitoring and connectivity platform for a more complete picture of the brain. O3 may help clinicians monitor cerebral oxygenation of adult and pediatric patients in situations in which pulse oximetry alone may not be fully indicative of the oxygen in the brain.
@MasimoInnovates || #Masimo
References
1. Benkreira A, Beaubien-Souligny W, Denault A, and Mailhot T. Postoperative delirium and hepatic venous congestion: a prospective study. Proceedings from the 2018 IARS Annual Meeting, Chicago, Illinois. #1470.
2. Ibala R, Hahm, E, Maher S, Nebreda M, Garcia E, Nozari A, and Akeju O. Decreased EEG alpha/beta power during sevoflurane general anesthesia is associated with pre-existing cognitive impairment. Proceedings from the 2018 IARS Annual Meeting, Chicago, Illinois. #1173.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www2.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo O3® and SedLine®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo O3 and SedLine, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Investigates the Utility of Masimo SpHb® in Post-operative Red Blood Cell (RBC) Transfusion Best Practices
Lisbon, Portugal – April 19, 2018 – Masimo (NASDAQ: MASI) announced today the findings of an abstract presented at the 2018 Annual Meeting of the Network for the Advancement of Patient Blood Management, Haemostatis and Thrombosis (NATA), in which researchers investigated the utility of Masimo noninvasive and continuous hemoglobin (SpHb®) in supporting and enhancing red blood cell (RBC) transfusion best practices as part of post-operative patient blood management (PBM).1
-

Masimo Radical-7® with SpHb® and RD rainbow SET™ Sensor
In the study, Prof. Baricchi and colleagues in the Transfusion Medicine Unit, AUSL-IRCCS of Reggio Emilia and at the Department of Medicine and Surgery at the University of Parma, Italy, sought to evaluate the appropriateness of post-operative RBC transfusion over a three-year period (2013-2015), before and after implementation of a patient blood management (PBM) program. The PBM policy consisted of three months of training followed by the introduction of noninvasive, continuous point-of-care (POC) monitoring of patient hemoglobin using Masimo SpHb on Masimo Radical-7® Pulse CO-Oximeters®. An initial audit of RBC transfusion appropriateness was conducted prior to the introduction of the PBM policy, on 168 patients. A final audit, after the policy was in place, was conducted on 205 patients. To determine transfusion appropriateness, investigators used the guidelines established by the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI).
The researchers found that prior to the introduction of the PBM policy, 37.7% of RBC transfusions were appropriate. After the introduction of the PBM policy, including use of noninvasive and continuous hemoglobin monitoring with Masimo SpHb, they found 65.4% of RBC transfusions were appropriate, a significant increase in transfusion appropriateness.
The researchers concluded that, "In our experience, the PBM strategies introduced improved RBC transfusion appropriateness in the post-operative period. We believe that our PBM policy and introduction of POC testing are a valuable support for the healthcare workers in the transfusion decision-making process. This enhancement of transfusion appropriateness implies clinical and managerial advantages, such as reduced transfusion-related risks, optimization of the health care resources and reduction of the costs."
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
Reference
1. Baricchi R, Marraccini C, Merolle L, Berni P, Bonini A, Mazzi A, Pertinhez T.A., and Di Bartolomeo E. Patient blood management: transfusion appropriateness in the postoperative period. Proceedings from the 2018 NATA Annual Meeting, Lisbon, Portgual. #P30.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www2.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces CE Marking of Rad-97™ Pulse CO-Oximeter® with Integrated NomoLine™ Capnography
Continuous Oxygenation and Ventilation Monitoring with Upgradeable rainbow® Parameters in a Compact, Standalone Device
Irvine, California – April 10, 2018 – Masimo (NASDAQ: MASI) announced today the CE marking of the Rad-97™ Pulse CO-Oximeter® with integrated NomoLine™ capnography. With this clearance, Rad-97 is now available both within and outside the United States in three configurations: Rad-97, Rad-97 with integrated noninvasive blood pressure, and now Rad-97 with NomoLine capnography. Rad-97 offers Masimo noninvasive and continuous monitoring, through Measure-through Motion and Low Perfusion™ SET® pulse oximetry, upgradeable rainbow® technology, and NomoLine capnography in a compact, standalone monitor that incorporates advanced customizability, connectivity, and device integration capabilities.
-
Masimo Rad-97™ Pulse CO-Oximeter® with NomoLine™ Capnography
Rad-97 with NomoLine capnography features an integrated ISA™ CO2 module with NomoLine sampling lines for sidestream capnography, with an adapter for intubated patients – meeting continuous monitoring and capnography needs in a single device. Rad-97 with capnography displays continuous end-tidal carbon dioxide (EtCO2) values with numeric, trend, and waveform viewing options, as well as fractional concentration of inspired carbon dioxide (FiCO2) and respiration rate. With both integrated capnography and Masimo acoustic respiration rate (RRa®) available on a single device, clinicians have the flexibility of choosing the most appropriate respiration monitoring method for each patient.
Rad-97 combines its portable, compact form factor with a high-resolution, multi-touch color display that allows clinicians to easily customize the device for each monitoring use case – bringing rainbow SET™ measurements and capnography measurements to care areas where a small footprint or high portability is desired. Rad-97 features built-in enterprise WiFi capability, allowing it to connect wirelessly to supplemental patient monitoring systems including Masimo Patient SafetyNet™*, facilitating automatic data transfer to hospital electronic medical record (EMR) systems. The easy-to-use, intuitive interface helps to simplify charting workflows for vital sign monitoring and patient data capture.
"We're delighted to bring the full family of Rad-97 products to markets outside the United States," said Joe Kiani, Founder and CEO of Masimo. "We believe Rad-97, with its versatility and customizability, will play an especially important role in helping hospitals improve their monitoring capabilities with convenient, easy-to-use device and technology solutions – ultimately helping to improve patient care and safety."
Rad-97, Rad-97 with noninvasive blood pressure, and Rad-97 with NomoLine capnography have received FDA 510(k) clearance and are also available in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at http://www2.masimo.com/evidence/featured-studies/feature/.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Rad-97™ and NomoLine™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-97 and NomoLine, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces UniView™
UniView Aggregates and Displays Patient Data From Multiple Devices to Minimize Clinician Cognitive Overload and Maximize Patient Safety
Irvine, California – April 2, 2018 – Masimo (NASDAQ: MASI) announced today the release of UniView™, an integrated display of real-time data and alarms from multiple Masimo and third-party devices, designed to reduce clinician cognitive overload and improve patient safety. UniView promotes data sharing and team coordination among multiple clinicians.
In operating rooms (ORs), in intensive care units (ICUs), and in other areas which involve multiple clinical disciplines, clinicians are often unable to simultaneously view data from all of the various medical devices in use, resulting in information that remains siloed among, for example, anesthesiologists, surgeons, and nurses. In addition, the plethora of displays and user interfaces adds to cognitive overload that can cause clinician burnout and suboptimal patient care.
-

Masimo UniView™
UniView solves these problems by bringing together data from a variety of sources – such as patient monitors, ventilators, anesthesia gas machines, and IV pumps – and providing a supplementary display for them, clearly organized, on one or more large, central monitors, so that all clinicians can simultaneously view and act upon the same, comprehensive real-time patient status and historical trends. Visual alarm indicators, relayed from the connected devices, help clinicians recognize patient distress and target areas for immediate focus. UniView is suitable for any area where comprehensive, logically organized, and timely data are key to supporting good clinical care.
UniView also provides tailored, use-case-specific screen layouts which optimize the presentation of advanced and integrated parameters, trend data, and waveforms for a variety of clinical scenarios, making it a versatile solution in many environments. For example:
- • In Overview layout, view monitoring data from all connected point-of-care and therapeutic devices including waveforms and alarms, for an overview of patient status.
- • In Hemodynamics layout, view trend data for noninvasive hemoglobin (SpHb®), pleth variability index (PVi®), and pulse rate to aid in visualizing patient status over time.
- • In Oxygenation layout, view ventilator waveforms alongside noninvasive trended hemoglobin (SpHb) and oxygen saturation (SpO2) to monitor a patient’s oxygenation status.
- • In Sedation layout, view high-fidelity EEG waveforms, patient state index (PSi), and anesthesia machine data to monitor a patient’s sedation.
In addition, hospitals will be able customize the view as preferred and even take advantage of individual customization using Masimo MyView™ technology.
Joe Kiani, Founder and CEO of Masimo, said, "We are answering clinicians' calls for logical, clinically transformational cockpits for the collaborative ORs and ICUs of the future. UniView is a great example of Masimo’s ongoing commitment to automating patient care, whether it's making pulse oximeters accurate when you need that accuracy most, creating new noninvasive monitoring technologies such as SpHb, or in this case, unsoiling data, enhancing connectivity, and communicating patient data as effectively and efficiently as possible, so that clinicians can focus more on their patients. We look forward to introducing additional enhancements soon – helping to bring automation and connectivity to the next level."
UniView builds on the success of Kite™, which projects data from Root® on a larger screen, by aggregating data from all of the connected devices in a room, including third-party ones, so that a supplemental display of all monitoring data can be viewed by all. UniView works in conjunction with Masimo Iris Gateway™ and Patient SafetyNet™* connectivity platforms. Complementing UniView, the recently announced Masimo Replica™ allows clinicians to view similar monitoring data for multiple patients, as well as view and respond to alarms and alerts, all from a smart phone, regardless of location.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo UniView™, Iris Gateway™, and Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Replica, Iris Gateway, and Patient SafetyNet, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Investigates the Performance of Masimo SpHb® During Acute Bleeding and After Fluid Resuscitation
Neuchatel, Switzerland – March 12, 2018 – Masimo (NASDAQ: MASI) announced today the findings of a recently published study in which researchers at Cairo University in Egypt evaluated the accuracy and trending of Masimo noninvasive and continuous hemoglobin (SpHb®), referenced to an invasive laboratory hemoglobin (Lab-Hb) measurement, during acute bleeding and after fluid resuscitation.1
-

Masimo Radical-7® with SpHb®, PVi®, and RD rainbow SET™ Sensor
In the study, Dr. Adel and colleagues sought to investigate the performance of SpHb in different volume statuses and perfusion states. They utilized intraoperative data from 70 patients scheduled for major orthopedic procedures with anticipated major blood loss. SpHb, as well as Masimo PVi® and Pi, were measured using a Masimo Radical-7® Pulse CO-Oximeter®. Lab-Hb was measured using a Beckman Coulter LH 750 analyzer. SpHb and Lab-Hb were recorded at three times – a baseline reading five minutes after endotracheal intubation, after major bleeding, and after fluid resuscitation – resulting in 210 time-matched readings, which were divided into fluid responsive and fluid non-responsive samples and low and high perfusion index samples.
Using Bland-Altman analysis, the researchers found "excellent correlation" between Lab-Hb and SpHb (r=0.938). In addition, they reported "excellent accuracy with moderate levels of agreement," as noted below, for various sample sets:
| Sample set | Mean bias | Limits of agreement |
|---|---|---|
| All | 0.01 | -1.33 to 1.34 g/dL |
| Fluid non-responsive | -0.08 | -1.27 to 1.11 g/dL |
| Fluid responsive | 0.09 | -1.36 to 1.54 g/dL |
| High Pi | 0.01 | -1.34 to 1.31 g/dL |
| Low Pi | 0.04 | -1.31 to 1.39 g/dL |
The researchers also noted that polar plot analysis (angular bias of -4°) showed "good trending ability for SpHb as a follow-up monitor."
The investigators concluded that, "SpHb showed excellent correlation with Lab-Hb in fluid responders, fluid non-responders, low-Pi, and high-Pi states. Despite a favorable mean bias of 0.01 g/dL for SpHb, the relatively wide levels of agreement (-1.3 to 1.3 g/dL) might limit its accuracy. SpHb showed good performance as a trend monitor."
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
Reference
1. Adel A, Awada W, Abdelhamid B, Omar H, Dayem O, Hasanin A, and Rady A. Accuracy and trending of noninvasive hemoglobin measurement during different volume and perfusion statuses. J Clin Mon. 2018. https://doi.org/10.1007/s10877-018-0101-z.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.


Masimo Announces Replica™
Replica Allows Clinicians to Remotely Monitor Patients from Anywhere in Real Time
Las Vegas, Nevada – March 6, 2018 – Today at the 2018 HIMSS Annual Conference Masimo (NASDAQ: MASI) announced the release of Replica™, an application for smart phones and tablets that works in conjunction with Masimo Patient SafetyNet™*, a supplemental remote monitoring and clinician notification system. Replica allows clinicians to view continuous monitoring data for multiple patients, as well as view and respond to alarms and alerts, all from their smart phones, regardless of location.
With Patient SafetyNet, continuous monitoring data are available at a central viewing station. Replica builds on this capability by delivering continuous monitoring data and intelligent alarm notifications to remote clinicians on smart phones. Replica displays data relayed from connected bedside Masimo and third-party devices, such as ventilators and patient monitors. Replica also allows the display of high fidelity data, such as waveforms, in real time. Clinicians can also access up to 96 hours of historical data, aiding assessment of potential deterioration over time. The collation of data from multiple disparate sources in a single location provides clinicians with a more complete picture of patient status at a glance.
-
Masimo Replica™
Replica features intelligent two-way alarm and alert notification technology, derived from Patient SafetyNet's existing capabilities, that offers significant advantages over systems which send out rudimentary, one-way notifications. Replica extends Patient SafetyNet’s intelligence by routing and escalating detailed, color-coded alarm and alert notifications to active mobile devices, reaching on-duty and available clinicians. Clinicians can respond to notifications from the app – choosing to accept or forward – and see if other clinicians have already responded. By combining detailed monitoring data with intelligent notification, Replica helps to improve clinical collaboration, promoting informed, timely response and effective clinical coordination.
Joe Kiani, Founder and CEO of Masimo, said, "Given the high patient-to-clinician ratios common in many areas, such as the medical-surgical floor, there is an increasing need for continuous remote visibility into patient status. Patient SafetyNet, combined with the power of SET® pulse oximetry, has been shown to save lives, reduce rapid response activations, and reduce costs in post-surgical wards.1-3 Replica takes the benefits of Patient SafetyNet to a new level by intelligently and reliably delivering valuable patient data and notifications to clinicians wherever they may be – thus helping them to respond and intervene as effectively and efficiently as possible."
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
2. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
3. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Replica™ and Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Replica and Patient SafetyNet, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Reports Fourth Quarter 2017 Financial Results
Q4 2017 Highlights (compared to Q4 2016):
- • Total revenue, including royalty and other revenue, increased 22.9% to $225.2 million
- • Product revenue increased 13.4% to $199.2 million
- • 54,100 SET® and rainbow SET™ oximeters were shipped
- • GAAP net income of $45.6 million, or $0.82 per diluted share. Non-GAAP net income of $41.9 million, or $0.75 per diluted share.
- • GAAP net income of $0.4 million, or $0.01 per diluted share, which included a non-recurring charge of $43.5 million, or
$0.78 per diluted share, related to the Tax Cuts and Jobs Act of 2017. Non-GAAP net income was $40.0 million, or $0.72
per diluted share.
Irvine, California, February 27, 2018 Masimo (NASDAQ: MASI) today announced its financial results for the fourth quarter ended December 30, 2017.
Fourth Quarter 2017 Results:Fourth quarter total revenue, including royalty and other revenue, increased 22.9% to $225.2 million, up from $183.2 million for the fourth quarter of 2016. Product revenues for the fourth quarter of 2017 increased 13.4% to $199.2 million, compared to $175.7 million for the fourth quarter of 2016.
The Company’s worldwide direct product revenue, which accounted for 87.1% of total product revenue, increased by 13.5% to $173.4 million in the fourth quarter of 2017 compared to $152.8 million for the fourth quarter of 2016. OEM sales, which accounted for 12.9% of total product revenue, increased 12.6% to $25.8 million in the fourth quarter of 2017 compared to $22.9 million for the fourth quarter of 2016. Revenue from sales of Masimo rainbow® products increased 42.5% to $24.0 million in the fourth quarter of 2017 compared to $16.9 million for the fourth quarter of 2016.
GAAP net income for the fourth quarter of 2017 was $0.4 million, or $0.01 per diluted share, compared to GAAP net income of $215.3 million, or $3.97 per diluted share, in the fourth quarter of 2016. Included in the fourth quarter 2017 earnings per share was a non-recurring charge of $43.5 million, or $0.78 per diluted share, related to the Tax Cuts and Jobs Act of 2017 (2017 Tax Act) that was signed into law on December 22, 2017. The fourth quarter 2016 earnings per share included a non-recurring gain of $270.0 million, or $3.43 per diluted share, related to the Philips Settlement Agreement. On a non-GAAP basis, the Company reported fourth quarter net income of $40.0 million, or $0.72 per diluted share, compared to net income of $24.6 million, or $0.45 per diluted share, in the fourth quarter of 2016.
During the fourth quarter of 2017, the Company shipped approximately 54,100 SET® pulse oximeters and rainbow SET™ Pulse CO-Oximeters®, excluding handheld and finger oximeters. Masimo estimates its worldwide installed base of oximeters to be 1,591,000 units as of December 30, 2017, up 5.8% from 1,504,000 units as of December 31, 2016.
As of December 30, 2017, total cash and cash equivalents were $315.3 million compared to $306.0 million as of December 31, 2016. During 2017, the Company repurchased approximately 0.8 million shares of common stock at a total cost of $68.3 million.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, “Our fourth quarter and full year 2017 results clearly illustrate the strength of our technology and hence our business as we once again exceeded expectations for product revenues and earnings. We realized another record high for shipments of our SET® Pulse Oximeters which reached 54,100 for the quarter and exceeded 200,000 for the year, a significant milestone for the Company. We anticipate rising adoption of our technologies as more care providers appreciate the value they provide for patients.”
2018 Financial GuidanceThe Company provided the following estimates for its full year 2018 guidance:
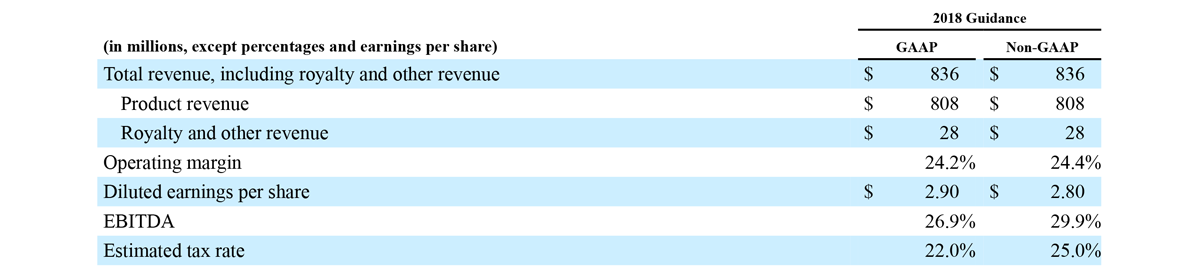
- • Total revenue, including royalty and other revenue, of $836 million;
- • Product revenue increasing 9% to $808 million compared to $741 million for 2017;
- • GAAP diluted earnings per share of $2.90, an increase of approximately 23% compared to $2.36 for 2017; and
- • Non-GAAP diluted earnings per share of $2.80, an increase of approximately 14% compared to $2.45 for 2017.
Supplementary Non-GAAP Financial Information
For additional non-GAAP financial details, please visit the Investor Relations section of the Company’s website at www.masimo.com to access Supplementary Financial Information.
Non-GAAP Financial Measures
The non-GAAP financial measures contained herein are a supplement to the corresponding financial measures prepared in accordance with U.S. generally accepted accounting principles (GAAP). The non-GAAP financial measures presented exclude the items described below. Management believes that adjustments for these items assist investors in making comparisons of periodto- period operating results or that these items are not indicative of the Company’s on-going core operating performance. These non-GAAP financial measures have certain limitations in that they do not reflect all of the costs associated with the operations of the Company’s business as determined in accordance with GAAP. Therefore, investors should consider non-GAAP financial measures in addition to, and not as a substitute for, or as superior to, measures of financial performance prepared in accordance with GAAP. The non-GAAP financial measures presented by the Company may be different from the non-GAAP financial measures used by other companies.
The Company has presented the following non-GAAP measures to assist investors in understanding the Company’s core net operating results on an on-going basis: (i) non-GAAP net income, (ii) non-GAAP diluted earnings per share, (iii) non-GAAP gross profit, (iv) non-GAAP operating income and (v) adjusted EBITDA. These non-GAAP financial measures may also assist investors in making comparisons of the Company’s core operating results with those of other companies. Management believes non-GAAP gross profit, non-GAAP operating income, non-GAAP net income, non-GAAP net income per diluted share and adjusted EBITDA are important measures in the evaluation of the Company’s performance and uses these measures to better understand and evaluate our business.
The non-GAAP financial measures reflect adjustments for the following items, as well as the related income tax effects thereof:
Acquisition-related costs, including depreciation and amortization.
Depreciation and amortization related to the revaluation of assets and liabilities (primarily intangible assets, property, plant and equipment adjustments, inventory revaluation, lease liabilities, etc.) to fair value through purchase accounting related to value created by the seller prior to the acquisition rather than ongoing costs of operating our core business. As a result, we believe that exclusion of these costs in presenting non-GAAP financial measures provides management and investors a more effective means of evaluating historical performance and projected costs and the potential for realizing cost efficiencies within our core business. Depreciation and amortization related to the revaluation of acquisition related assets and liabilities will generally recur in future periods.
Litigation damages, awards and settlements.
In connection with litigation proceedings arising in the course of our business, we have recorded expenses as a defendant in such proceedings in the form of damages, as well as gains as a plaintiff in such proceedings in the form of litigation awards and settlement proceeds; most recently in connection with our November 2016 settlement agreement with Koninklijke Philips N.V. We believe that exclusion of these expenses and gains is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these expenses and gains are generally unrelated to our core business and/or infrequent in nature.
Realized and unrealized gains or losses from foreign currency transactions.
We are exposed to foreign currency gains or losses on outstanding foreign currency denominated receivables and payables related to certain customer sales agreements, product costs and other operating expenses. As the Company does not actively hedge these currency exposures, changes in the underlying currency rates relative to the U.S. Dollar may result in realized and unrealized foreign currency gains and losses between the time these receivables and payables arise and the time that they are settled in cash. Since such realized and unrealized foreign currency gains and losses are the result of macro-economic factors and can vary significantly from one period to the next, we believe that exclusion of such realized and unrealized gains and losses are useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. Realized and unrealized foreign currency gains and losses are likely to recur in future periods.
Excess tax benefits from stock-based compensation.
Current authoritative accounting guidance requires that excess tax benefits or costs recognized on stock-based compensation expense be reflected in our provision for income taxes rather than paid-in capital. Since we cannot control or predict when stock option awards will be exercised or the price at which such awards will be exercised, the impact of such guidance can create significant volatility in our effective tax rate from one period to the next. We believe that exclusion of these excess tax benefits or costs is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. These excess tax benefits or costs will generally recur in future periods as long as we continue to issue equity awards to our employees.
Tax impacts that may not be representative of the ongoing results of our core operations.
The Tax Cuts and Jobs Act of 2017 (2017 Tax Act) was signed into law in December 2017, and became effective January 1, 2018. The 2017 Tax Act included a number of changes to existing U.S. federal tax law impacting businesses including, among other things, a permanent reduction in the corporate income tax rate from 35% to 21%, a one-time transition tax on the “deemed repatriation” of cumulative undistributed foreign earnings as of December 31, 2017 and changes in the prospective taxation of the foreign operations of U.S. multinational companies. We believe that exclusion of the tax charges related to the 2017 Tax Act is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that this tax charge is unrelated to our core business and non-recurring in nature.
Fourth Quarter 2017 Actuals versus Fourth Quarter 2016 Actuals:
- 1.The impact results from tax effecting the pre-tax adjustments above at the effective statutory rate, taking into account any
discrete items applicable to the pre-tax non-GAAP adjustments. - 2.The 2017 Tax Act resulted in an unfavorable charge of $43.5 million in the fourth quarter of 2017. Included in this charge
was $9.0 million related to the re-measurement of deferred taxes due to the reduction in the federal corporate tax rate, $28.0
million related to the transition tax on the deemed repatriation of foreign earnings, and $6.5 million related to estimated foreign
withholding taxes, net of estimated foreign tax credit benefit, and state income taxes related to the Company’s tentative
decision to repatriate up to $180.0 million of accumulated undistributed foreign earnings to the U.S. in the future. The amount
recognized is a provisional estimate and subject to change, possibly materially, due to, among other things, refinements of
the Company’s calculations, changes in interpretations and assumptions the Company has made or additional guidance issued
by the U.S. Treasury, Securities and Exchange Commission or Financial Accounting Standards Board.
Full Year 2017 Actuals versus Full Year 2018 Guidance:
- 1. Estimated effective tax rate of 22% applied to GAAP earnings and 25% applied to non-GAAP earnings.
- 2.The impact results from tax effecting the pre-tax adjustments above at the effective statutory rate, taking into account any discrete items applicable to the pre-tax non-GAAP adjustments.
- 3.The 2017 Tax Act resulted in an unfavorable charge of $43.5 million in the fourth quarter of 2017. Included in this charge
was $9.0 million related to the re-measurement of deferred taxes due to the reduction in the federal corporate tax rate, $28.0
million related to the transition tax on the deemed repatriation of foreign earnings, and $6.5 million related to estimated foreign
withholding taxes, net of estimated foreign tax credit benefit, and state income taxes related to the Company’s tentative
decision to repatriate up to $180.0 million of accumulated undistributed foreign earnings to the U.S. in the future. The amount
recognized is a provisional estimate and subject to change, possibly materially, due to, among other things, refinements of
the Company’s calculations, changes in interpretations and assumptions the Company has made or additional guidance issued
by the U.S. Treasury, Securities and Exchange Commission or Financial Accounting Standards Board.
- 1. Other (income)/expense consists primarily of interest (income)/expense and net foreign currency (gains)/losses.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be available online from the investor relations page of the Company’s website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 4776719. After the live webcast, the call will be available on Masimo’s website through May 30, 2018. In addition, a telephonic replay of the call will be available through May 9, 2018. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 4776719.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the Company debuted Masimo SET® Measure-through Motion and Low Perfusion® pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®) and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate and perfusion index (PI). In 2014, Masimo introduced Root™, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface. Masimo is also taking an active leadership role in mobile health applications (mHealth) with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at http://www.masimo.com/evidence/featured-studies/feature/.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year GAAP and non-GAAP 2018 total, product, royalty and other revenues, earnings per diluted share, operating margin, EBITDA, and estimated tax rate, and our long-term outlook; demand for our products; anticipated revenue and earnings growth; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management’s current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow SET™ products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors’ assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the “Risk Factors” section of our most recent periodic reports filed with the Securities and Exchange Commission (“SEC”), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact: Eli Kammerman
Phone: (949) 297-7077
Email: ekammerman@masimo.com
Media Contact: Irene Paigah
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI and ORI are trademarks or registered trademarks of Masimo Corporation.
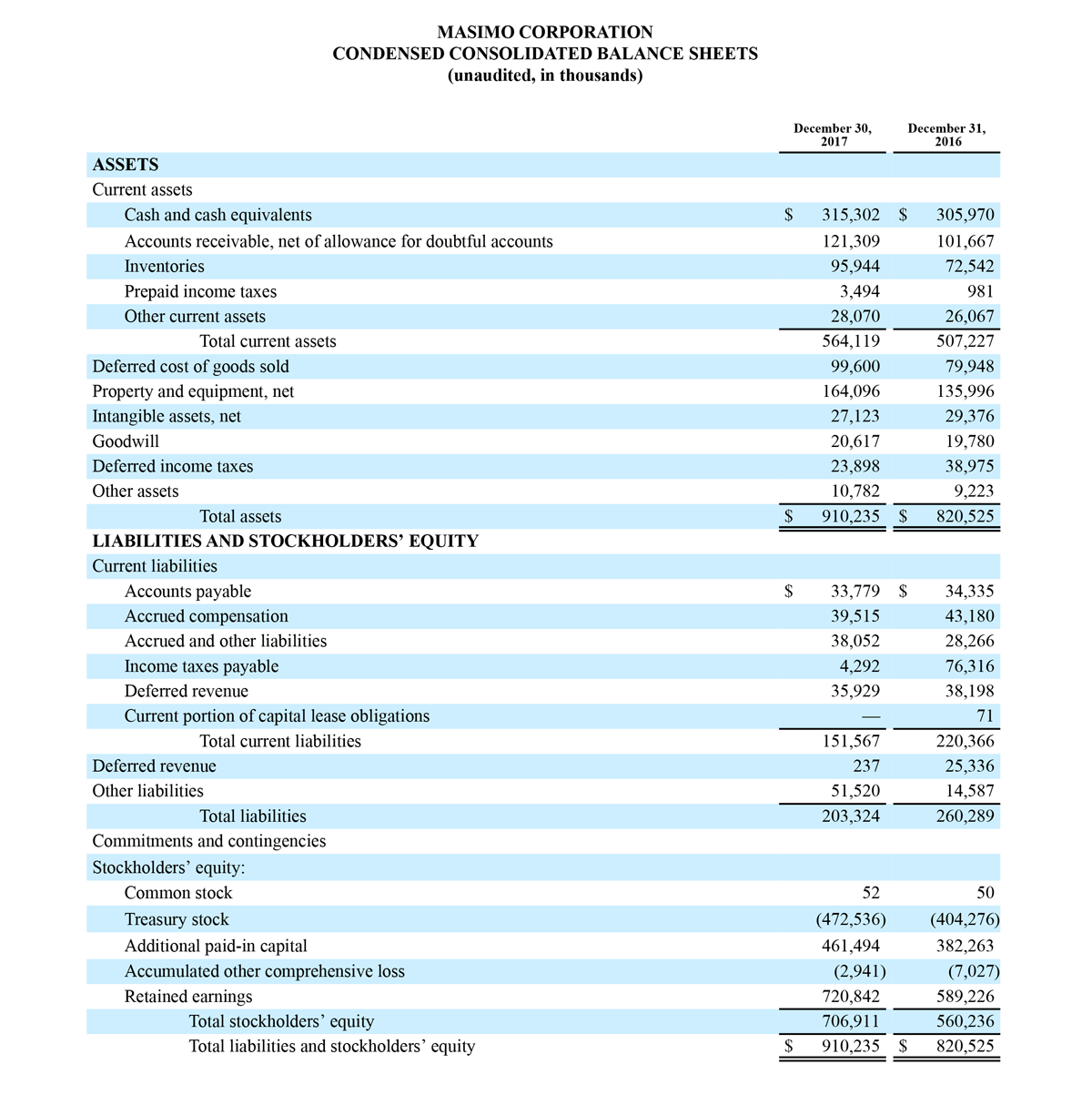

Leading Nephrology Care Center in India Adopts Masimo Technologies Across Continuum of Care
Bangalore, India – February 26, 2018 – Masimo (NASDAQ: MASI) announced today that NU Hospitals, a leading nephrology care center in India, has standardized on Masimo technologies across the continuum of care. NU Hospitals have entered into a strategic partnership with Masimo to adopt technologies in their operating rooms (ORs), intensive care units (ICUs), dialysis beds, and general wards at both of their facilities in Bangalore.
"We have been evaluating various technologies that could help our clinicians improve patient safety and at the same time help reduce the cost of care. Masimo, with its innovative range of solutions – including remote monitoring systems, depth of sedation monitoring, and noninvasive monitoring of hemoglobin and fluid responsiveness – will help us accomplish these twin objectives," said Dr. Prasanna Venkatesh, Pediatric Urologist & Managing Director, NU Hospitals.
NU Hospitals’ ORs will be equipped with Masimo Root® Patient Monitoring and Connectivity Hubs and Radical-7® Pulse CO-Oximeters®. The monitors will include noninvasive, continuous hemoglobin monitoring (SpHb®), a Masimo rainbow® parameter, and, via Masimo Open Connect® (MOC-9®) technology, SedLine® brain function monitoring.
In critical care areas and general wards, patients at NU Hospitals will now also be continuously monitored using a variety of bedside devices such as Root with Noninvasive Blood Pressure and Temperature and Rad-97™ Pulse CO-Oximeters. Measurements will include SpHb, SET® Measure-through Motion and Low Perfusion™ pulse oximetry, vital signs such as blood pressure and temperature, and, for dialysis patients, pleth variability index (PVi®) monitoring to assist in optimizing fluid management.
The bedside devices will be connected to Masimo Patient SafetyNet™*, a supplemental remote monitoring and clinician notification system which allows patient monitoring data to be accessed from a central viewing station. When changes occur in measured values that may indicate deterioration in a patient’s condition, Patient SafetyNet automatically sends wireless alerts directly to clinicians, wherever they may be, allowing clinicians to respond quickly to patients in potential distress. In addition, Patient SafetyNet will automate the transfer of patient data, including vital signs, early warning scores (EWS), and other physiological parameters, directly to NU Hospitals' electronic medical record (EMR) system.
Dr. Venkatesh Krishnamoorthy, Chairman and Sr. Consultant Urologist, NU Hospitals, commented, "We believe that every hospital patient should be continuously monitored. The automatic integration of data into our EMR and reduction in the manual recording of patient vitals will help us reduce transcription errors, build efficiency, and improve workflow."
"We are excited to work with NU Hospitals and help them take better care of their patients with our advanced monitoring technologies," said Jon Coleman, President of Worldwide Sales, Professional Services, and Medical Affairs, Masimo. "We applaud NU Hospitals for advancing the standard of care in their region."
Bharat Monteiro, Country Manager for Masimo in India, added, "We are hopeful that more hospitals in India will adopt such advanced technologies across the continuum of care to help improve patient safety and reduce the cost of care."
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect® (MOC-9®) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Masimo Root®, Radical-7®, SpHb®, MOC-9®, SedLine®, Rad-97™, SET®, PVi®, and Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root, Radical-7, SpHb, MOC-9, SedLine, Rad-97, SET®, PVi, and Patient SafetyNet, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Evaluates the Performance of Masimo SpHb® in Monitoring Pediatric Trauma Patients
Irvine, California – February 22, 2018 – Masimo (NASDAQ: MASI) announced today the findings of a recently published study in which researchers at Michigan State University evaluated the performance of noninvasive hemodynamic monitoring (NIHM) using Masimo noninvasive and continuous hemoglobin (SpHb®), as compared to invasive laboratory hemoglobin (LabHb) monitoring, in clinically stable pediatric trauma patients with solid organ injury.1
In the study, Dr. Welker and colleagues utilized data from 21 patients under 18 years of age who had experienced blunt trauma, with a mean injury severity score of 16.6. Their hemodynamic status was assessed using physical examination and vital signs in conjunction with periodic LabHb monitoring per normal institutional pediatric trauma guidelines. In addition, NIHM using Masimo SpHb was measured continuously using a Masimo Radical-7® Pulse CO-Oximeter®, and SpHb values were recorded at multiple times to correspond with LabHb blood draws.
-
Masimo Radical-7® with SpHb®
Using Bland-Altman analysis, the researchers calculated an average bias of 0.80 g/dL between SpHb and LabHb, with a 95% confidence interval of +3.94 g/dL to -2.33 g/dL. They noted that, "Measurement trends were highly correlated in patients with stable hemoglobin levels and those requiring blood transfusion."
The investigators concluded that, "NIHM demonstrated clinically acceptable accuracy when following hemoglobin trends in the defined pediatric trend patient population. Slight variances between NIHM and LabHb values were occasionally noted, but did not affect clinical management. Continuous NIHM represents a potentially valuable adjunct to traditional laboratory hemoglobin monitoring."
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
Reference
1. Welker E, Novak J, Jelsma L, Koehler T, Davis A, DeCou J, and Durkin E. Continuous hemoglobin monitoring in pediatric trauma patients with solid organ injury. J Pediatr Surg. 2018. https://doi.org/10.1016/j.jpedsurg.2017.12.015.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Evaluates the Performance of Masimo PVi® as a Predictor of Fluid Responsiveness in Mechanically Ventilated ICU Patients
Neuchatel, Switzerland – February 12, 2018 – Masimo (NASDAQ: MASI) announced today the findings of a recently published study in which researchers at Bülent Ecevit University in Zonguldak, Turkey compared two noninvasive methods of predicting fluid responsiveness in mechanically ventilated patients in the intensive care unit (ICU): Masimo PVi® (pleth variability index, measured noninvasively and continuously using SET® pulse oximetry sensors) and dIVC (distensibility index of inferior vena cava, measured noninvasively by radiologists using an ultrasound machine and probe).1
In the study, Drs. Pişkin and Öz sought to compare the performance of the two methods by enrolling 72 mechanically ventilated patients and taking various measurements before and after passive leg raising (PLR). In addition to PVi (measured with Masimo Radical-7® Pulse CO-Oximeters®) and dIVC (measured by a radiologist using Esaote MyLab 30), central venous pressure (CVP) and cardiac index (CI) were measured. Patients who exhibited a greater than 15% increase in CI attributable to the PLR maneuver were classified as volume responders, and those with less than 15% or no change, as nonresponders.
-
Masimo Radical-7® with PVi®
The researchers found that Masimo noninvasive and continuous PVi, at a threshold value of >14%, provided 95% sensitivity and 81.2% specificity (p<0.001, AUC = 0.939 (0.857-0.982)), which was statistically significant. Ultrasound, noninvasive dIVC, at a threshold value of >23.8%, provided 80% sensitivity and 87.5% specificity (p<0.001, AUC = 0.928 (0.842-0.975)), which was also statistically significant. The invasive method, CVP, at a threshold value of ≤7 mmHg, provided 70% sensitivity and 53.1% specificity to predict fluid responsiveness (p=0.066, Area Under the Curve = 0.622 (0.500-0.724)), which was not statistically significant.
The investigators noted that "our results show that noninvasively assessed PVi and dIVC were good predictors of fluid responsiveness after PLR in ICU patients on mechanical ventilation. By contrast, the invasively assessed CVP was a poor predictor of fluid responsiveness as a static variable of cardiac preload." They concluded that, "Both PVi and dIVC may be used to identify the fluid responsiveness of all ICU patients undergoing continuous treatment linked to mechanical ventilation; both methods are easily applied, noninvasive, and can be performed at the bedside."
Reference
1. Pişkin Ö and Öz I. Accuracy of pleth variability index compared with inferior vena cava diameter to predict fluid responsiveness in mechanically ventilated patients. Medicine. (2017) 96:47(e8889).
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo PVi®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo PVi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces CE Marking of Eve™ CCHD Newborn Screening Application for the Rad-97™ Pulse CO-Oximeter®
Neuchatel, Switzerland – February 7, 2018 – Masimo (NASDAQ: MASI) announced today the CE marking of Eve™, a critical congenital heart disease (CCHD) newborn screening application, for the Rad-97™ Pulse CO-Oximeter®. Eve combines the power of Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry with a pre-ductal to post-ductal synchronization algorithm designed to reduce calculation errors.
Eve, also available on the Radical-7® Pulse CO-Oximeter, simplifies the CCHD screening process by providing visual instructions, animations, an automatic synchronization algorithm, and a detailed, easy-to-interpret display of screening results. The ability to label results with unique patient identifiers for both mother and newborn facilitates intuitive session management and seamless electronic charting. Eve also allows clinicians to incorporate perfusion index into screening, which has been shown to increase sensitivity to the detection of CCHD in infants with pathologically low perfusion.1
-

Masimo Rad-97™ with Eve™
CCHD affects approximately 3 newborns per 1000 live births2 and requires intervention soon after birth to prevent significant morbidity or mortality; later detection in infants also increases the risk of brain damage.3 In a study of 39,821 infants, CCHD screening sensitivity increased from 63% with physical exam alone to 83% with physical exam and Masimo SET® pulse oximetry.4 In a study of 122,738 infants – the largest CCHD screening study to date – CCHD screening sensitivity increased from 77% to 93% with the combined use of Masimo SET® and clinical assessment.5
"Multiple studies have shown that Masimo SET® pulse oximetry can help improve CCHD screening, helping to save many newborns' lives while reducing the cost of care," commented Joe Kiani, Founder and CEO of Masimo. "Eve builds on the powerful benefits of SET® by transforming pulse oximeters into intuitive screening tools which can help clinicians perform the crucial work of screening newborns in an easy-to-follow and intuitive application."
Eve has not obtained FDA clearance and is not available in the United States. Radical-7 and Rad-97 have obtained FDA clearance and are available, without Eve, in the United States.
References
1. de-Wahl Ganelli et al. Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction. Acta Paediatr. 2007 Oct;96(10):1455-1459.
2. Hoffman JL et al. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890-1900.
3. 2011 Legislative Report; State of Maryland, Department of Health and Mental Hygience, State Advisory Council on Hereditary and Congenital Disorders. Recommendations on Implementation of Screening for Critical Congenital Hearth Disease in Newborns. Page 7.
4. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
5. Zhao et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET®, Eve™, Rad-97™, and Radical-7®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET®, Eve, Rad-97, and Radical-7, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo to Report Fourth Quarter and Full Year 2017 Financial Results after Market Close on Tuesday, February 27
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., January 30, 2018 - Masimo (NASDAQ: MASI) announced today that it will release fourth quarter and full year 2017 financial results for the period ended December 30, 2017, after the market closes on Tuesday, February 27, 2018. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Micah Young, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 9789137. After the live webcast, the call will be available on Masimo's website through March 27, 2018. In addition, a telephonic replay of the call will be available through March 6, 2018. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 9789137.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Eli Kammerman
Phone: (949) 297-7077
Email: ekammerman@masimo.com
Media Contact:
Irene Paigah
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces FDA Clearance of Next Generation SedLine® Brain Function Monitoring
Irvine, California – January 29, 2018 – Masimo (NASDAQ: MASI) announced today FDA clearance of Next Generation SedLine® brain function monitoring. SedLine helps clinicians monitor the state of the brain under anesthesia with bilateral acquisition and processing of four leads of electroencephalogram (EEG) signals. Next Generation SedLine features an enhanced signal processing engine, driving a variety of performance improvements and helping give clinicians a more complete picture of the brain.
-
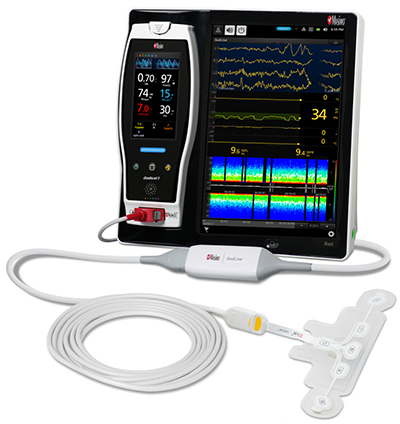
Masimo Root® with Next Generation SedLine® Brain Function Monitoring
Next Generation SedLine provides an enhanced Patient State Index (PSi) – a processed EEG parameter related to the effect of anesthetic agents – based on its enhanced signal processing engine.
In addition, Next Generation SedLine displays an enhanced, Multitaper Density Spectral Array (DSA), developed by Dr. Emery Brown, MD, PhD, and Dr. Patrick Purdon, PhD, Director and Associate Director, respectively, of the Neuroscience Statistics Research Lab at Massachusetts General Hospital. The DSA represents activity in both sides of the brain. When displaying a Multitaper DSA, EEG data are transformed into the frequency domain, and may provide a better display of EEG features.
Joe Kiani, Founder and CEO of Masimo, said, "Masimo is committed to innovation in the brain function monitoring space. With the improved performance offered by its enhanced signal processing capabilities, we expect Next Generation SedLine to impact brain function monitoring just as SET® impacted pulse oximetry."
When used with the RD SedLine™ EEG sensor, Next Generation SedLine can be used simultaneously with O3® regional oximetry on the Root® patient monitoring and connectivity platform for even more insight into a patient's brain. O3 may help clinicians monitor cerebral oxygenation in situations in which pulse oximetry alone may not be fully indicative of the oxygen in the brain.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Next Generation SedLine® and O3®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Next Generation SedLine and O3, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Investigates the Clinical Utility of ORi™, Masimo Oxygen Reserve Index™, in Obese Patients
Neuchatel, Switzerland – January 22, 2018 – Masimo (NASDAQ: MASI) announced today the findings of an abstract presented at the annual meeting of the Society for Technology in Anesthesia (STA) in Miami, Florida. In the study, researchers at the UC Davis School of Medicine evaluated the potential clinical utility of Masimo Oxygen Reserve Index™ (ORi™) as an early warning of impending arterial hemoglobin desaturation in obese patients.1 This is the first published research investigating the utility of ORi in this particular population group.
ORi is a relative indicator of a patient's oxygen reserve in the moderate hyperoxic region (partial pressure of oxygen in arterial blood [PaO2] in the range of 100 to 200 mmHg). As an "index" parameter with a unit-less scale between 0 and 1, ORi can be trended and has optional alarms to notify clinicians of changes in a patient’s oxygen reserve.
In the prospective, observational study, Dr. Ayala and colleagues analyzed data from 36 adult patients with BMI between 30 and 40 kg/m2 who were scheduled for elective surgical procedures requiring general anesthesia and endotracheal intubation. The patients' ORi values were measured using a Masimo Root® Patient Monitoring and Connectivity Platform with Radical-7® Pulse CO-Oximeter®. The researchers recorded the time elapsed from the start of ORi alarming (triggered by decrease in the absolute value and rate of change in ORi) to 98% oxygen saturation, and considered this interval to be the average increase in warning time provided by ORi.
The researchers found that among the patients, the average time from the start of ORi alarming to 98% oxygen saturation was 42 ± 49 seconds (ranging from 5 to 255 seconds). Excluding two outliers, the average increase in warning time provided by ORi was 33 ± 23 seconds (ranging from 5 to 107 seconds).
The researchers concluded that the study "demonstrates the ability of ORi to provide advanced warning of arterial desaturation as an adjunct to SpO2 in this high risk patient population. This additional warning time can potentially translate to improved patient safety by allowing earlier calls for help, assistance from a more experienced person, or modification of airway management. For this analysis we defined the advance warning to end at 98% SpO2, with a defined trigger for intervention at 94% SpO2.”
In another study, researchers at Children’s Medical Center in Dallas, Texas concluded that ORi could provide clinicians with a median of 31.5 seconds advanced warning of impending desaturation in pediatric patients with induced apnea after pre-oxygenation.2
UC Davis received funding from Masimo for the ORi study.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
References
1. Ayala S, Singh A, Applegate R, and Fleming N. Oxygen Reserve Index: Utility as Early Warning of Desaturation in Morbidly Obese Patients. Proceedings from the 2018 STA Annual Meeting, Miami, FL.
2. Szmuk P et al. Oxygen Reserve Index A Novel Noninvasive Measure of Oxygen Reserve–A Pilot Study. Anesthesiology. 4 2016, Vol. 124, 779-784. doi:10.1097/ALN.0000000000001009.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo ORi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo ORi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces FDA Clearance for Home Use of Rad-97™
Rad-97 Comes with Advanced Communication Capabilities to Facilitate Telehealth
Irvine, California – January 9, 2018 – Masimo (NASDAQ: MASI) announced today FDA clearance for home use of the Rad-97™ Pulse CO-Oximeter®. Rad-97 offers Masimo noninvasive and continuous monitoring, through Measure-through Motion and Low Perfusion™ SET® pulse oximetry and upgradeable rainbow® technologies, in a compact, standalone monitor that combines advanced connectivity and communication capabilities for telehealth with an interface easily customized for use at home.
-

Masimo Rad-97 Pulse CO-Oximeter with Optional Integrated Camera
With an ever increasing number of patients receiving care at home, there is a growing need for high-quality home monitoring and telehealth equipment. Rad-97, in addition to SET® pulse oximetry and advanced parameter monitoring technology through rainbow® noninvasive monitoring, is available with optional integrated blood pressure and capnography measurements. Its innovative communication capabilities allow monitoring data from a variety of third-party Bluetooth®-enabled devices used at home, including thermometers, weight scales, and glucometers, to seamlessly transfer to Rad-97 – and from there to anywhere in the world, in real time. The optional integrated camera allows remote clinicians to interact with patients over live audio and video. Rad-97 brings hospital-grade technology to the home in a single, integrated device that is a monitoring, connectivity, and telecommunications hub.
-

Patient surveillance using Masimo Patient SafetyNet
With its built-in enterprise WiFi capability, Rad-97 has the ability to connect wirelessly from the home to supplemental patient monitoring systems in the hospital, including Masimo Patient SafetyNet™*, allowing clinicians to remotely observe patient status while facilitating automatic data transfer to hospital electronic medical record (EMR) systems. Rad-97 can trend and store data for up to 96 hours.
With its intuitive touchscreen interface, Rad-97 is easy to use for clinicians and non-clinicians alike, and can be easily customized to meet the needs of home users. The device can be configured in Home mode, which streamlines basic utility, giving home users access to applicable settings and messages, while hiding others and locking alarm settings, minimizing the chances of inadvertent interaction.
Joe Kiani, Founder and CEO of Masimo, said, "Rad-97 is well-suited for a variety of care settings, now including home. Facilitated by its powerful connectivity and telehealth capabilities, Rad-97 allows clinicians to remotely interact with patients, as well as monitor their status and spot trends from afar through automated data collection and transfer."
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Rad-97™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-97, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces CE Marking and Release of Oxygen Reserve Index™ rainbow Lite Sensors
Neuchatel, Switzerland – January 2, 2018 – Masimo (NASDAQ: MASI) announced today the CE marking and release of RD rainbow Lite SET™ sensors, which enable the monitoring of Masimo Oxygen Reserve Index™ (ORi™) and RPVi™, an improved PVi® that allows clinicians to assess fluid responsiveness noninvasively and continuously1 at a fraction of the cost of invasive methods, and at a fraction of the cost of rainbow® sensors. rainbow Lite sensors utilize twice as many wavelengths of light as SET® sensors, allowing rainbow Lite sensors to provide ORi and RPVi along with Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry.
-
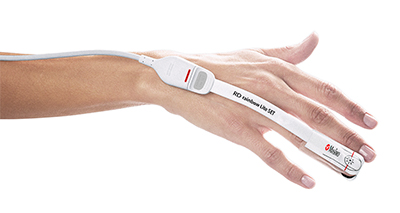 Masimo RD rainbow Lite SET™ Sensor
Masimo RD rainbow Lite SET™ Sensor
ORi is the first noninvasive and continuous parameter to provide insight into a patient’s oxygen reserve in the moderate hyperoxic range. In conjunction with SET® pulse oximetry, ORi may provide advanced warning of impending desaturation, which may allow clinicians to intervene sooner. For example, in a study of 25 pediatric patients undergoing general anesthesia with orotracheal intubation, researchers found that ORi helped clinicians identify impending desaturation a median of 31.5 seconds before noticeable changes in oxygen saturation (SpO2) occurred.2 In another recent study of 106 adult patients scheduled for surgery with arterial catheterization and intraoperative blood gases analyses, researchers found a significant relationship between change in PaO2 and change in ORi.3 In addition, ORi may provide insight into oxygen reserve when titrating patients who are receiving supplemental oxygen.4
Joe Kiani, Founder and CEO of Masimo, said, "rainbow Lite sensors allow clinicians, who depend on powerful SET® pulse oximetry technology, to augment their patient monitoring with ORi and newly introduced RPVi. Given the positive reception of ORi in available markets, and feedback from clinicians who see great value in the benefits of ORi monitoring, we are excited to make ORi and RPVi accessible via the cost-effective solution represented by RD rainbow Lite SET. Hospitals that standardize on RD rainbow Lite SET will pay only nominally more per sensor than for SET®."
Masimo RPVi is a multi-wavelength version of Pleth Variability Index (PVi). RPVi is designed to provide enhanced assessment of changes in fluid volume compared to PVi5, which has been shown in over 90 independent clinical studies to be as effective as invasive monitoring methods.6
With the addition of RD rainbow Lite SET, the RD family of sensors is now available in three levels of capability: RD SET, utilizing two wavelengths (2 LED) and featuring SET® pulse oximetry; RD rainbow Lite SET, which utilizes four wavelengths (4 LED) and adds the ability to measure ORi and RPVi; and RD rainbow SET, which utilizes over seven wavelengths (7+ LED) and enables the measurement of additional advanced noninvasive parameters such as SpHb® (total hemoglobin), SpCO® (carboxyhemoglobin), SpMet® (methemoglobin), and SpOC™ (oxygen content).
Like RD SET sensors, RD rainbow Lite SET sensors are designed to maximize patient comfort and optimize clinician workflow. The sensors are lightweight and have a flat, soft cable with smooth edges, so that they lie comfortably on a patient’s hand or foot. The sensors feature an intuitive sensor-to-cable connection, with tactile and audible feedback to ensure a proper connection, and have graphics printed on both sides to assist with application.
Devices with ORi and RPVi measurements and RD rainbow Lite SET sensors have not received FDA 510(k) clearance and are not available for sale in the United States.
References
1. Forget P, Lois F, de Kock M. Goal-Directed Fluid Management Based on the Pulse Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management. Anesth Analg. 2010 Oct;111(4):910-4.
2. Szmuk P, Steiner JW, Olomu PN, Ploski RP, Sessler DI, and Ezri T. Oxygen Reserve Index A Novel Noninvasive Measure of Oxygen Reserve—A Pilot Study. Anesthesiology. 4 2016, Vol. 124, 779-784. doi:10.1097/ALN.0000000000001009.
3. Applegate R, Dorotta I, Wells B, Juma D, and Applegate P. The Relationship Between Oxygen Reserve Index and Arterial Partial Pressure of Oxygen During Surgery. Anesth Analg. 2016 Sep; 123(3); 626-633.
4. Scheeren TWL, Belda FJ and Perel A. The oxygen reserve index (ORi): a new tool to monitor oxygen therapy. J Clin Monit Comput. 2017. doi:10.1007/s10877-017-0049-4.
5. Masimo data on file.
6. Published clinical studies on PVi can be found at www.masimo.com/en-GB/home/clinical-evidence/pleth-variability-index-pvi/.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo RD rainbow Lite SET™ sensors, ORi™, RPVi™, and PVi®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo RD rainbow Lite sensors, ORi, RPVi, and PVi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.