News & Media-2017
Home / 2017
2017
NEWS & MEDIA:

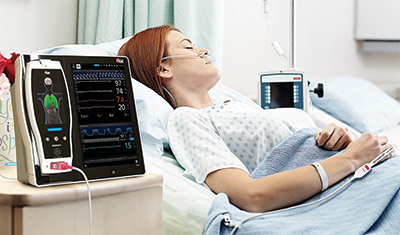
Artemis Hospital Becomes First in India to Adopt Masimo Patient SafetyNet™ Across All Hospital Care Areas
Supplemental Patient Surveillance System, in Conjunction with Masimo SET® and rainbow® Monitors, Provides Continuous Remote Monitoring 24 Hours a Day
Gurgaon, India – December 1, 2017 – Masimo (NASDAQ: MASI) announced today that Artemis Hospital, one of the leading multi-specialty hospitals in the Delhi-NCR region of India, is adopting Masimo Patient SafetyNet™*, a supplemental remote monitoring and clinician notification system, across all hospital care areas.
Patient SafetyNet, in conjunction with Masimo SET® pulse oximetry and rainbow® pulse CO-oximetry, will enable the hospital team to monitor key patient parameters and gain insight, via changes in patient status recorded by Masimo bedside monitors, into possible signs of patient deterioration. Artemis is the first hospital in India to offer such round-the-clock patient surveillance across all specialties. With Patient SafetyNet, Artemis hopes to achieve results similar to those achieved at Dartmouth-Hitchcock Medical Center in the United States: In 2016, the Medical Center, which had been using Masimo SET® pulse oximetry and Patient SafetyNet as part of a comprehensive alarm management strategy in all medical-surgical units for ten years, reported achieving a 50% reduction in unplanned ICU transfers and a 60% reduction in rescue events over those ten years, despite increases in patient acuity and occupany.1,2
"Artemis is renowned for its patient-centric care and we wanted to further augment it using technologies that can continuously monitor patients across different care areas," said Dr. Devlina Chakravarty, Executive Director of Artemis Hospitals. "Given our stringent standards for quality and state-of-the-art infrastructure, Patient SafetyNet was a natural fit."
Patient SafetyNet allows clinicians at a central station to review patient data continuously relayed from bedside monitoring devices. It also features a robust supplemental alarm notification and escalation process that relays notifications to clinicians wherever they may be in the hospital. If notifications remain unacknowledged, they are escalated to additional clinicians per a customizable protocol.
Dr. (Col.) Manjinder Singh Sandhu, Medical Director and Director-Cardiology, Artemis Hospitals, said, "Artemis has always been ahead of the industry in adopting initiatives and technologies that can improve patient care. Continuous supplemental remote monitoring in areas not usually monitored is one such initiative and we wanted a solution that can seamlessly integrate with our existing protocols and infrastructure. We found Masimo Patient SafetyNet to be one such system that offered both unmatched value and hassle-free integration."
Jon Coleman, President of Worldwide Sales, Professional Services, and Medical Affairs, Masimo, said, "Artemis is leading the way for patient safety in India with their commitment to monitor all of their patients all of the time. Continuous remote monitoring with SET® technology saves lives and precious resources. We are delighted to serve Artemis Hospital with our technology."
Bharat Monteiro, Country Manager, India and ISC, for Masimo, added, "We are in the process of altering the landscape of continuous and remote monitoring in India with our customized product offerings and unique value proposition. Masimo and Artemis share a commitment to patient care and we are excited to work with the Artemis team to help make a difference in care and satisfaction of their patients and clinicians."
Artemis care areas will be equipped with bedside monitoring devices such as the Radius-7® Pulse CO-Oximeter®, a Bluetooth- and WiFi-enabled wearable, tetherless monitor used in conjunction with the Root® Patient Monitoring and Connectivity Platform, as well as Rad-97™, Masimo’s most recent bedside pulse CO-oximeter. These devices feature SET® Measure-through Motion and Low Perfusion™ pulse oximetry technology to measure oxygen saturation, pulse rate, and perfusion index for all patients, with fewer of the false alarms3 that have made monitoring patients in less nurse-intensive areas impractical. A subset of patients will also be monitored using SpHb®, a Masimo rainbow® parameter that measures hemoglobin, continuously and noninvasively. Each floor will also use a cart-mounted Root with Noninvasive Integrated Blood Pressure and Temperature monitor to provide periodic spot-checking of vital signs and input for automated Early Warning Scores, built into Root. Patient data from all of these devices, in addition to being relayed to Patient SafetyNet, will be automatically integrated with the hospital’s electronic medical record (EMR) and hospital information system (HIS).
Artemis Hospital, established in 2007, is a 380-bed, state-of-the-art multi-specialty hospital located in Gurgaon, India, the first in Gurgaon to be accredited by the Joint Commission International (JCI) and the National Accreditation Board for Hospitals and Healthcare Providers (NABH).
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
2. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
3. Shah N et al. Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers. J Clin Anesth. 2012 Aug;24(5):385-91.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Patient SafetyNet, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces FDA Clearance and Worldwide Release of NomoLine™ Capnography Sampling Lines
Includes U.S. Release of NomoLine Airway Adapter Sets for Neonatal and Infant Patients
Irvine, California – November 27, 2017 – Masimo (NASDAQ: MASI) announced today FDA clearance and U.S. release of the full family of NomoLine™ capnography sampling lines. NomoLine sampling lines are available in more than 40 configurations of airway adapter sets and cannulas for use in a variety of clinical scenarios, including for intubated and non-intubated patients in both low and high humidity applications, for all patient populations, including neonatal patients. NomoLine capnography sampling lines are compatible with both Masimo and third-party OEM NomoLine monitors and enable hassle-free sidestream capnography and gas monitoring.
-
 Masimo Root® Patient Monitoring and Connectivity Platform with NomoLine™ Capnography
Masimo Root® Patient Monitoring and Connectivity Platform with NomoLine™ Capnography
NomoLine "no moisture" sampling technology eliminates many common problems associated with conventional sidestream gas sampling line systems. Incorporating a unique, patented polymer, NomoLine allows water in the sampling line to continuously evaporate into the surrounding air, while leaving oxygen, carbon dioxide, and anesthetic gases unaffected. This technology eliminates the need for water traps and issues related to their handling, as well as enabling extended monitoring time in high humidity applications, reducing the volume of disposables and the cost and waste associated with them.
NomoLine technology is designed for low-flow applications, with a very low sampling rate of 50 ml/min, supporting use on patients with low tidal volumes and high breath rates, common characteristics of neonatal patients. With functionality in any orientation, NomoLine provides a variety of sampling line options including nasal, nasal/oral, oxygen delivery, single nasal prong, and airway adapter sets. Soft, ergonomically curved cannulas help provide greater patient comfort.
NomoLine sampling lines are compatible with NomoLine ISA™ capnography modules, including ISA CO2, ISA AX+, and ISA OR+ for multigas monitoring. ISA modules are available on the expandable Masimo Root® Patient Monitoring and Connectivity Platform through Root's Masimo Open Connect™ (MOC-9™) ports, as well as on more than 70 OEM monitors, including those from Spacelabs, Schiller, Ortivus, Siare, and Edan. In addition, the Rad-97™ Pulse CO-Oximeter® is now available with an integrated module allowing direct connection to NomoLine sampling lines.
"With NomoLine, we've developed innovative moisture-wicking technology – and then applied that breakthrough to an entire line of cannulas, including neonatal airway adapter sets. Masimo continues to automate noninvasive monitoring with solutions such as NomoLine that are reliable and easy to use," said Joe Kiani, Founder and CEO of Masimo.
Rad-97 and neonatal NomoLine and ISA products are currently available in the United States only.
-
 NomoLine High Humidity Nasal Cannula, High Humidity Airway Adapter Set for Infant/Neonatal Patients, and Low Humidity Oral/Nasal Cannula with Oxygen Delivery
NomoLine High Humidity Nasal Cannula, High Humidity Airway Adapter Set for Infant/Neonatal Patients, and Low Humidity Oral/Nasal Cannula with Oxygen Delivery
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of NomoLine™ capnography. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including NomoLine capnography, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Supports World Pneumonia Day Efforts
"Every Breath Counts Coalition" Aims to Help Governments End Preventable Pneumonia Deaths in a Set of African Countries by 2030
Irvine, California – November 13, 2017 – In conjunction with World Pneumonia Day on Sunday, November 12, Masimo (NASDAQ: MASI) announced its continuing efforts to help combat the global burden of pneumonia.
Each year, it is estimated that 178,000 newborns and 773,000 children die from pneumonia, making it the leading global cause of death in children under five.1 Increasingly, the major pneumonia burden is in the first 2 years of life, with the problem being particularly challenging in low-resource settings such as sub-Saharan Africa.2,3
It is time for action in eliminating pneumonia. Today Masimo and its partners in the fight against pneumonia are debuting a documentary entitled United for Oxygen, examining the problem, available at this linkwhere you can watch the documentary, and ask for your assistance in spreading the word across the world.
Along with more than 30 other organizations, including UNICEF, Save the Children, The Newborn Foundation, The Bill & Melinda Gates Foundation, The Clinton Health Access Initiative, GSK, and Philips, Masimo is a founding member of the "Every Breath Counts Coalition," dedicated to eliminating all pneumonia-related deaths. Each member of the coalition brings unique expertise to the problem, with some working on pneumococcal vaccine coverage, some on access to medical oxygen, others on education, and, in Masimo's case, the use of SET® pulse oximetry, the most accurate and reliable pulse oximetry in the world;4 pulse oximetry is often included in relevant pneumonia clinical screening protocols. As part of a grant from The Bill & Melinda Gates Foundation, Masimo has developed Rad-G™, a low-cost pulse oximeter that can be used as part of pneumonia screening, amongst other applications.
Joe Kiani, Founder and CEO of Masimo, said, "Eliminating pneumonia will take everyone's unified efforts. Together we can bend the curve and eliminate preventable childhood deaths from pneumonia, so no child has to fight to breathe. How can you help? Join the 'Every Breath Counts Coalition.'"
Rad-G is currently not available for sale in the United States, Canada, or the E.U.
References
1. Miles, Carolyn. Partnering to Fight Pneumonia, The ‘Forgotten Killer’ of Children. Huffington Post. 31 October 2017. https://www.huffingtonpost.com/entry/59f8d6d6e4b0b7f0915f6271.
2. Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhea. Lancet 2013; 381: 1405–16.
3. Green and Kolberg. Neonatal pneumonia in sub-Saharan Africa, Pneumonia (2016) 8:3 DOI 10.1186/s41479-016-0003-0.
4. Published clinical studies on Masimo SET® pulse oximetry can be found at http://www.masimo.com/home/clinical-evidence/masimo-set-pulse-oximetry/.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET® and Rad-G™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET® and Rad-G, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces Distribution of Rad-57® Pulse CO-Oximeters® by The Jeffrey Lee Williams Foundation to First Responders
Foundation Awards Devices to York County EMS Departments to Assist in Monitoring for Presence of Carbon Monoxide
Rock Hill, York County, South Carolina – November 7, 2017 – Masimo (NASDAQ: MASI) announced today that The Jeffrey Lee Williams Foundation has distributed 20 Masimo Rad-57® Pulse CO-Oximeters® to EMS departments in York County, South Carolina, with the majority going to Piedmont Medical Center EMS. The Foundation purchases equipment that assists in monitoring for the presence of carbon monoxide (CO) and donates it to the community and local fire and first response agencies. The Rad-57s were awarded to the departments at an event on November 6.
-

The Jeffrey Lee Williams Foundation Presents Masimo Rad-57® Pulse CO-Oximeters® to Members of the Piedmont Medical Center EMS (York County, South Carolina)
The Jeffrey Lee Williams Foundation was founded in 2013 to honor the tragic loss of Jeannie Williams’ son Jeffrey at the age of 11 to CO poisoning. Its mission is to help prevent CO poisoning by facilitating the distribution and placement of equipment to detect and monitor for CO. Currently, the Foundation is working in York County to sponsor the distribution of 2,000 CO alarms among all 18 fire districts in York County for installation in residential homes, the distribution of 45 “always-on” CO monitors among fire departments and EMS departments, and the distribution of the 20 Masimo Rad-57s to first responders and EMS departments.
CO poisoning is a leading cause of unintentional poisoning deaths in the United States.1 In addition, just one severe CO exposure event nearly doubles the risk of premature death, and consistent CO exposure may cause long-term heart and brain damage.2,3 Even mild levels of CO circulating in the blood rob the heart and brain of oxygen, which can cause mental confusion, leading to poor decision making and increasing the risk of heart disease or stroke – two conditions that account for nearly 50% of on-duty firefighter deaths.4,5
Rad-57 provides oxygen saturation, pulse rate, and perfusion index measurements using SET® Measure-through Motion and Low Perfusion™ pulse oximetry. In addition, it includes SpCO®, a noninvasive rainbow® parameter, to measure the amount of carboxyhemoglobin in red blood cells; carboxyhemoglobin forms after exposure to CO. Noninvasive SpCO monitoring may lead to the identification of elevated CO levels that might otherwise go undetected in front-line settings.
Amber Williams, Co-founder of The Jeffrey Lee Williams Foundation, South Carolina, “We are thrilled to offer Piedmont Medical Center EMS and two other rescue squads the Rad-57s! These devices will provide quick, noninvasive, objective data to responders to assist in the identification and treatment of CO exposure in our community.”
Jeannie Williams, Co-founder, added, “Thank you Masimo for your support as we work to help others in Jeffrey’s memory.”
Joe Kiani, Founder and CEO of Masimo, said, “We are saddened by the loss of life due to CO poisoning and delighted to help support The Jeffrey Lee Williams Foundation’s important work. We hope that Rad-57 and SpCO technology can help to identify elevated CO levels in York County EMS responders, firefighters, and civilians.”
SpCO is intended to be used to monitor CO levels in the blood and is not intended to be used as the sole basis for making diagnosis or treatment decisions related to carbon monoxide poisoning. SpCO monitoring is not intended to replace laboratory blood testing; blood samples should be analyzed by laboratory instruments prior to clinical decision making.
References
1. Carbon Monoxide Exposures, United States, 2000-2009. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6030a2.htm.
2. Hampson NB et al. Increased long-term mortality among survivors of acute carbon monoxide poisoning. Crit Care Med. 2009; 37(6):1941-47.
3. Bledsoe BE. The heart dangers of CO: Understanding cardiovascular risks to responders from CO exposure. J Emerg Med Svcs. 2007; 32:54-59.
4. Jakubowski G. The Invisible Incidents: How to respond to CO alarms. FireRescue Magazine. 2004; 22(11):52-55.
5. Bledsoe BE. The Perils of CO. FireRescue Magazine. September 2005.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
The Jeffrey Lee Williams Foundation
Jeannie and Amber Williams
info@jeffreysfoundation.org
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


San Diego Bankruptcy Court Rules Sotera Employees Misappropriated Masimo Trade Secrets
San Diego, California – October 16, 2017 – Masimo (NASDAQ: MASI) announced today that the bankruptcy court in San Diego has issued final judgment, holding that Sotera Wireless employees misappropriated Masimo trade secrets. The misappropriation stems from two former Masimo employees, James Welch and David Hunt, copying thousands of confidential Masimo documents, and using Masimo's trade secrets to benefit Sotera. The Court found clear and convincing evidence that willful and malicious misappropriation exists. The court also found that the actions of Welch and Hunt were "despicable conduct," and that they "consciously disregarded Masimo's rights."
The Court has permanently enjoined Sotera from retaining, disclosing, disseminating or using confidential Masimo documents, originating from either James Welch's or David Hunt's Masimo computers. The Court also enjoined Sotera, until September 16, 2021, from including Mr. Welch in Sotera's Design Control process, including preparing the Customer Needs Document, the Design Input Requirements documentation, the Software Requirements Specification or User Story, implementing software design and the Software Design Specification, the Design Review, the Verification process, the Validation process, developing schematics and other production specifications, and preparing the design history file. David Hunt had not been working at Sotera since 2015. Masimo understands that Mr. Welch is also no longer employed by Sotera.
This case was originally the subject of an action in the Superior Court of Orange County filed in May 2013 against Welch, Hunt and Sotera. This portion of that case was tried in the bankruptcy court in San Diego after Sotera filed for Chapter 11 bankruptcy in 2016. Thus, the San Diego ruling concerned the misappropriation of Masimo's trade secrets by Sotera. The remaining portion of the Superior Court action against Welch and Hunt remains pending.
Joe Kiani, Founder and CEO of Masimo, said, "I am sad that we even had to pursue this case. These were trusted employees. I hope all members of Masimo’s team will adhere to Masimo’s guiding principles of 'remaining faithful to your promises and responsibilities, being driven by fascination and accomplishment, not power and greed, making every day as fun as possible, making themselves better each year, and, doing what is best for patient care.' We believe these guiding principles are critical not only to our success, but to our integrity and humanity."
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 17 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com


Masimo Announces U.S. Release of Trace™ Data Visualization and Reporting Tool
Irvine, California – October 5, 2017 – Masimo (NASDAQ: MASI) announced today the U.S. release of Trace™, patient data visualization and reporting software designed for Masimo Root® and Radical-7® monitors.
Trace is the first data visualization and reporting software compatible with the full capabilities of the Masimo Root Patient Monitoring and Connectivity Platform, including Radical-7 and Radius-7® Pulse CO-Oximeters®, Root with integrated noninvasive blood pressure and temperature, and connected MOC-9™ modules such as SedLine® brain function monitoring, ISA™ and ISA OR+ capnography, and O3® regional oximetry.
"Trace adds a valuable and powerful visualization resource to clinical toolkits," said Joe Kiani, Founder and CEO of Masimo. "With its unique versatility and customizability and with access to all of Masimo’s advanced measurement technologies, Trace offers clinicians the ability to review and focus on the patient data patterns that matter most for each case, in the format that provides the most insight."
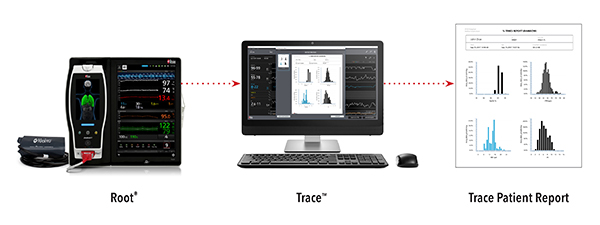
Clinicians who are interested in reviewing overnight sleep studies and who review six-minute walk patient data can benefit from Trace’s ability to rapidly generate standard oximetry reports, as well as customized oximetry reports that also include acoustic respiration rate (RRa®) and end-tidal carbon dioxide (EtCO2) data. Clinicians and researchers can download and evaluate patient data involving advanced rainbow SET™ parameters and capnography, SedLine, and O3 measurements. Trace can display patient data from all of these technologies on the same time axis, facilitating clinical case review. The ability to annotate and store data helps clinicians build easily accessible case histories.
Trace can communicate with Masimo devices via high-speed wired or wireless connections, with the ability to download up to 96 hours of patient data in seconds, aiding workflow efficiencies. Trace can provide analytics such as the determination of the minimum, maximum, and mean values for each measurement, the percentage of time spent at defined parameter thresholds, threshold crossing counts, and the duration of desaturation events. Visual tools such as trend graphs, histograms, and event annotations are also supported. Data can be tagged with notes, labeled with identifiers, and cropped and adjusted to allow clinicians to focus on segments and items of interest, from which a PDF report can be generated or CSV file exported.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Trace™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Trace, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces Full Market Release of rainbow Acoustic Monitoring Sensor RAS-45
Irvine, California – September 28, 2017 – Masimo (NASDAQ: MASI) announced today the full market release of RAS-45, an adhesive adult and pediatric acoustic respiration sensor for rainbow Acoustic Monitoring® (RAM™). RAS-45 offers the same performance as the currently available RAS-125c sensor but in a smaller size, with more flexible adhesive.
-
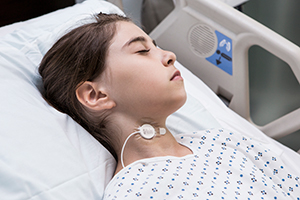
Masimo rainbow Acoustic Monitoring® RAS-45 Acoustic Respiration Sensor
RAM noninvasively and continuously measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer, such as Masimo's RAS-125c and now RAS-45, that is applied to the patient's neck area. Using acoustic signal processing that leverages Masimo Signal Extraction Technology (SET®), the respiratory signal is separated and processed to display continuous respiration rate (RRa®) and respiratory waveform, with the option to listen to the sound of breathing from the acoustic sensor.
RRa has been shown to be accurate1,2, easy-to-use1, easy-to-tolerate1,3, and reliable1, and has also been shown to enhance patient compliance with respiration monitoring. In a study comparing pediatric patient tolerance of sidestream capnography with a nasal cannula to respiration rate monitoring with an RAS-125c acoustic sensor, 15 out of 40 patients removed the cannula, while only one removed the acoustic sensor.3 In a study of 98 patients consciously sedated during upper gastrointestinal endoscopy, researchers found that RRa monitoring with the RAS-125c sensor more accurately assessed respiration rate than capnography using end-tidal carbon dioxide (EtCO2) measurement or impedance pneumography.2 RAS-45 maintains the same performance parameters, range, and accuracy specification as RAS-125c.
-
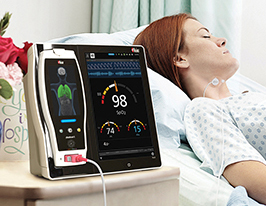
Masimo Root® Patient Monitoring and Connectivity Platform with rainbow Acoustic Monitoring® RAS-45 Acoustic Respiration Sensor
With its smaller size, RAS-45 is well suited for monitoring pediatric patients and patients with shorter necks. The RAS-45 adhesive is transparent, lighter, and more flexible than the RAS-125c adhesive. Like RAS-125c, RAS-45 operates with Masimo MX technology boards to measure RRa, display the acoustic respiration wave form, and optionally allow clinicians to listen to the sound of breathing. Both sensors are for adult and pediatric patients who weigh more than 10 kg.
Joe Kiani, Founder and CEO of Masimo, commented, "RAM harnesses the power of our breakthrough signal processing technology and applies it to a respiratory measurement derived from the sound of breathing. With the addition of the RAS-45 sensor, RRa is now a more convenient and comfortable measurement for clinicians and patients – especially children."
Continuous monitoring of respiration rate can be helpful in cases such as sedation-based procedures and post-surgical patients receiving patient-controlled analgesia for pain management. The Anesthesia Patient Safety Foundation (APSF) and The Joint Commission recommend continuous oxygenation and ventilation (respiration) monitoring for all patients receiving opioid-based pain medications.4,5
References
1. Macknet MR et al. Accuracy and Tolerance of a Novel Bioacoustic Respiratory Sensor in Pediatric Patients. Anesthesiology. 2007;107:A84 (abstract).
2. Goudra BG et al. Comparison of Acoustic Respiration Rate, Impedance Pneumography and Capnometry Monitors for Respiration Rate Accuracy and Apnea Detection during GI Endoscopy Anesthesia. Open J Anesthesiol. 2013;3:74-79.
3. Patino M et al. Accuracy of Acoustic Respiration Rate Monitoring in Pediatric Patients. Paediatr Anaesth. 2013 Sep 3.
4. Stoelting, RK et al. APSF newsletter. 2011. www.apsf.org.
5. The Joint Commission Sentinel Event Alert. Issue 49, August 8, 2012. www.jointcomission.org.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo RAS-45, RAM™, and RRa®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo RAS-45, RAM, and RRa, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com


Masimo Announces FDA Clearance and Full Market Release of Rad-97™
Pulse CO-Oximeter®
Irvine, California – September 18, 2017 – Masimo (NASDAQ: MASI) announced today FDA 510(k) clearance and full market release of Rad-97™ Pulse CO-Oximeter®, including configurations with integrated NomoLine™ capnography and noninvasive blood pressure (NIBP) measurement from SunTech Medical. Rad-97 offers Masimo noninvasive and continuous monitoring, through Measure-through Motion and Low Perfusion™ SET® pulse oximetry and upgradeable rainbow® technologies, in a compact, standalone monitor that incorporates advanced customizability, connectivity, and device integration capabilities.
"We're excited to bring the easy-to-use, compact Rad-97 to the US," said Joe Kiani, Founder and CEO of Masimo. "Rad-97 brings together our core SET® and rainbow® technologies with advanced, workflow-enhancing connectivity solutions. We believe it will be an indispensable addition to many healthcare environments, including lower-acuity settings in hospitals, alternate care, and telehealth."
-

Masimo Rad-97™ Pulse CO-Oximeter® (center)
Rad-97 with Integrated Noninvasive Blood Pressure (left)
Rad-97 with Integrated NomoLine™ Capnography (right)
Rad-97 combines its portable, compact form factor with a high-resolution, multi-touch color display that allows clinicians to easily customize the device for each monitoring use case — bringing rainbow SET™ measurements to care areas where a small footprint or high portability is desired. Users can also rapidly configure the device to accommodate different patient populations using customizable profiles. A rechargeable battery lasting approximately four hours allows Rad-97 to be used in situations where extended operation without access to AC power is needed. An optional roll stand allows for tetherless device transport, offering additional flexibility in situations where space is limited.
Rad-97 features built-in enterprise WiFi capability, allowing it to connect wirelessly to supplemental patient monitoring systems including Masimo Patient SafetyNet™*, facilitating automatic data transfer to hospital electronic medical record (EMR) systems. The easy-to-use, intuitive interface helps to simplify charting workflows for vital sign monitoring and patient data capture. Rad-97 is also compatible with existing nurse call systems. Data from extended monitoring sessions, such as sleep studies, can be rapidly downloaded via USB, Ethernet, or WiFi.
Rad-97 also supports point-to-point Bluetooth® wireless connections with compatible devices, such as thermometers, glucometers, and weight scales, allowing patient data to be seamlessly transferred to Rad-97 and connected upstream systems. Rad-97 will also be available with an optional camera that will provide a high-resolution video feed, as well as audio, to the Patient SafetyNet view-station. The camera-equipped Rad-97 will allow patients and clinicians to communicate remotely with compatible Patient SafetyNet software, making it well-suited as a point-of-care device for telehealth.
Rad-97 with capnography features an integrated ISA™ CO2 module with NomoLine sampling lines for sidestream capnography, with an adapter for intubated patients — meeting continuous monitoring and capnography needs in a single device. Rad-97 with capnography displays continuous end-tidal carbon dioxide (EtCO2) monitoring with numeric, trend, and waveform viewing options, as well as fractional concentration of inspired carbon dioxide (FiCO2) and respiration rate. NomoLine capnography reduces delays in respiration rate measurement in both low and high ranges, accurately measures respiration rate, and reduces the incidence of filter line occlusions, as a result of NomoLine moisture wicking technology.
With Rad-97 NIBP, oscillometric blood pressure is available in three NIBP measurement modes: spot-check, automatic interval (which measures blood pressure routinely, at a desired interval), and stat interval (which continually measures blood pressure for a desired duration). An integrated port allows clinicians to connect a blood pressure cuff inflation hose directly to Rad-97; the port is compatible with both disposable and reusable cuffs, for adult, pediatric, and neonatal patients.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Newborn Foundation and Masimo Partnership Reaches 52,000 Babies and 1,000 Health Workers through Newborn Screening Initiative in Support of Every Woman Every Child
BORN Project convenes public health stakeholders to highlight key program successes at three-year mark, reinforcing global call to action to support universal newborn pulse oximetry screening as a tool to significantly reduce newborn mortality and drive treatment infrastructure in low-resource settings
Sichuan Province, China – September 14, 2017 – Masimo (NASDAQ: MASI) and the Newborn Foundation today announced that the BORN Project, their joint commitment to the United Nations’ Every Woman Every Child (EWEC) initiative, has now screened 52,000 newborns across 40 delivery sites in China for critical congenital heart disease (CCHD), pneumonia, and sepsis. In addition, 1,000 health workers and public health staff have now been trained in neonatal pulse oximetry screening through the BORN project, which is also marking its third anniversary.
These progress updates will be discussed at a UN General Assembly event in New York City on Tuesday, September 19, "Harnessing the Power of Technology and Partnerships to Combat Newborn Mortality." The announcement comes on the heels of news that the National Health and Family Planning Commission, People’s Republic of China, and the Chinese CDC have convened a formal national committee to establish country-wide implementation and screening protocols for universal newborn pulse oximetry screening.
The BORN (Birth Oximetry Routine for Newborns) Project, developed by the Newborn Foundation in partnership with Masimo, aligns with public health initiatives with the goal of reducing newborn mortality from CCHD, pneumonia, and sepsis in pilot regions. It also provides substantive data to public health officials to demonstrate the importance of investment in sustainable universal newborn screening programs and improved access to follow-up care for fragile babies.
The project has provided the first large deployment of smart device-paired pulse oximeters for use on newborns. Training and educational tools, combined with the Masimo iSpO2® Rx smartphone- and tablet-paired pulse oximeter, teach families about the benefits of screening and allow healthcare workers at every level to screen babies and interpret screening results, helping to increase the rate of timely diagnosis and referrals.
"We have more than met the goals of the project since its launch, and seeing the impact on so many lives has been humbling," said Annamarie Saarinen, CEO of the Newborn Foundation. "The real impact has been in working in partnership with the government to bring this policy to fruition."
The BORN Project is conducted in collaboration with the China Office for Maternal and Child Health Surveillance, the China CDC, the Mianyang Health Bureau. It has been supported by Masimo, the Masimo Foundation, and the London-based Global Innovation Fund. An interim report on the findings of its impact of screening at hospitals in rural Sichuan Province was recently presented to the National Health and Family Planning Commission of the People’s Republic of China and Chinese CDC.
Established through an initial three-year, $50,000 commitment from Masimo in 2014 and subsequent $100,000 equipment donation, the BORN Project has now launched in the Philippines, where it is in the process of screening 72,000 newborns across 28 urban and rural hospitals, spanning all three island groups. The BORN Project is also expanding into India, Peru, Mexico, Bolivia, Pakistan, and Mongolia. The Newborn Foundation leads advocacy and implementation efforts, which focus on education, training and metrics on the benefits of routine pulse oximetry screening of newborns, as well as follow up diagnosis and treatment protocols.
"We must reframe how healthcare is delivered to the youngest and most fragile patients," said Joe Kiani, Founder and CEO of Masimo. "The work we are doing is already having a powerful impact in China and beyond. We are proud that Masimo SET®, with its proven clinical accuracy and reliability, is able to help save the lives of many newborns."
Every Woman Every Child
The Masimo and Newborn Foundation partnership was among the first commitment makers as part of the UN Secretary General’s Every Woman Every Child initiative. EWEC directly contributes to the Global Strategy for Women's, Children’s and Adolescents' Health and the success of the Sustainable Development Goals (SDGs).
With more than 50,000 rural and underserved babies impacted through their work thus far, Masimo and the Newborn Foundation, in collaboration with EWEC, are committed to addressing obstacles and tackling the unmet Millennium Development Goals for reducing mortality for children under the age of five, through the SDG initiatives.
iSpO2 Rx does not have 510(k) clearance and is not available for sale in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About Newborn Foundation
The Newborn Foundation conducts research, implements programs, and develops technology innovations that save lives and improve health outcomes for the newest, most vulnerable citizens of the world. The Foundation programmatically delivers these solutions to those who need them most and drives policies that integrate with public health initiatives to provide sustainable impact for babies and families.
About Every Woman Every Child
Launched in 2010 and led by the UN Secretary-General, the Every Woman Every Child movement aims to intensify national and international commitment and action by governments, the UN, multilaterals, private sector and civil society to keep women's, children's and adolescents' health and wellbeing at the heart of development. As a multi-stakeholder platform to operationalize the Every Woman Every Child Global Strategy for Women's, Children's and Adolescents' Health, the movement mobilizes partnerships and coordinated efforts across sectors to ensure that all women, children and adolescents not only survive, but also thrive to help transform the world. Since 2015, 62 country and around 150 multi-stakeholder commitments have been pledged in support of the EWEC Global Strategy, totaling nearly US$30 billion to deliver on the promises of a sustainable future for all. More information is available at http://www.everywomaneverychild.org.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo iSpO2® Rx. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo iSpO2 Rx, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Annamarie Saarinen
Newborn Foundation
Email: annamarie@newbornfoundation.org
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Study Evaluates Performance of Masimo iSpO2® Rx Smart Device-Paired Pulse Oximeter in Screening Newborns for Critical Congenital Heart Disease
Neuchatel, Switzerland – September 5, 2017 – Masimo (NASDAQ: MASI) announced today the findings of a recently published study in which researchers in Maastricht, Netherlands evaluated the performance of Masimo iSpO2® Rx in screening newborns for critical congenital heart disease (CCHD).1 iSpO2 Rx is a smart device-paired pulse oximeter featuring Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry technology.
CCHD affects approximately 2.5 to 3 newborns per 1000 live births2 and requires intervention soon after birth to prevent significant morbidity or mortality; later detection in infants also increases the risk of brain damage.3 In the study, Dr. Huizing and colleagues sought to evaluate the accuracy of iSpO2 Rx because of increasing trends toward out-of-hospital births and concerns about the costs of CCHD screening implementation in low-resource settings. The researchers surmised that a portable, low-cost pulse oximeter may be more practical in such settings than standalone hospital devices.
The researchers enrolled 201 infants in the study. CCHD screening was conducted 12-24 hours after birth by two independent teams, who were blinded to the other team's results. Each team took both preductal (right-hand) and postductal (either foot) oxygen saturation (SpO2) measurements to establish whether an infant passed, failed, or needed a repeat screening. One team measured SpO2 using a Masimo Radical-7® Pulse CO-Oximeter® equipped with disposable Masimo LNCS® sensors. The other team used a Masimo iSpO2 Rx equipped with a reusable M-LNCS™ YI sensor, connected to an Apple iPad Mini.
Applying Bland-Altman analysis to the preductal SpO2 values of the 201 screened infants, with Radical-7 as the reference device, the researchers calculated a mean bias of -0.08% ± standard deviation of 1.76%, with limits of agreement of -3.52% and 3.36%. For postductal SpO2 values, they calculated a mean bias of -0.11% ± standard deviation of 1.68%, with limits of agreement of -3.41% and 3.18%.
In addition, to evaluate the ability of iSpO2 Rx to measure low oxygen saturation, the researchers also enrolled a group of 12 infants admitted to the neonatal intensive care unit (NICU) with SpO2 lower than 95%. SpO2 was continuously monitored for 10 minutes, with readings recorded once per minute, and with one foot connected to the iSpO2 Rx and the other to a Philips IntelliVue MP70 monitor equipped with Masimo SET® technology. Using the Philips monitor as the reference device, the researchers calculated a mean bias of 0.01% ± standard deviation of 1.74%, with limits of agreement of -3.42% and 3.43%.
The researchers concluded that, "Our data suggest that CCHD screening with the Masimo iSpO2 Rx is feasible and accurate. The use of reliable smartphone-paired pulse oximeters may contribute to the extension of CCHD screening to home births and low resource settings." They also noted that, "The iSpO2 Rx demonstrated a high degree of agreement with the Masimo Radical-7, a hospital-grade pulse oximeter."
The device used in this study is iSpO2 Rx. The study did not use the iSpO2, which is intended as an exercise and wellness product and is not available for use on neonates. iSpO2 is not intended for CCHD screening or any other medical use.
iSpO2 Rx does not have 510(k) clearance and is not available for sale in the United States.
References
1. Huizing M, Villamor-Martinez-E, Chavagne I, Vanagt W, Spaanderman M, and Villamor E. Reliability and Validity of a Smartphone-Paired Pulse Oximeter for Screening of Critical Congenital Heart Defects in Newborns. Neonatology. 2017;112:324–329. DOI: 10.1159/000477294.
2. Hoffman JL et al. The incidence of congenital heart disease. J Am Coll Cardiol. 2002 Jun 19;39(12):1890-1900.
3. 2011 Legislative Report; State of Maryland, Department of Health and Mental Hygience, State Advisory Council on Hereditary and Congenital Disorders. Recommendations on Implementation of Screening for Critical Congenital Hearth Disease in Newborns. Page 7.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2017-18 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Study Evaluates Utility of Masimo SpHb® During High-Blood-Loss Oncosurgery
Neuchatel, Switzerland – August 28, 2017 – Masimo (NASDAQ: MASI) announced today the findings of a recently published study in which researchers in New Delhi, India evaluated the utility of Masimo SpHb®, noninvasive, continuous hemoglobin measurement, during oncosurgery on patients with high anticipated blood loss.1
In the study, Dr. Gupta and colleagues sought to evaluate the utility of SpHb measurements on patients undergoing oncosurgery because oncosurgeries “may be associated with large blood loss, requiring repeated haemoglobin estimation for deciding the need for intraoperative blood transfusion.” They enrolled 50 adult patients with anticipated blood loss of at least 20%. During surgery, the patients’ SpHb was continuously monitored using a Masimo Radical-7® Pulse CO-Oximeter®. The researchers obtained venous blood samples, which were analyzed using a Beckman Coulter analyzer (LabHb), at the following points: immediately after induction, when approximately 500 ml of blood loss was suspected, and just before reversal of the neuromuscular blockade.
A total of 137 paired (SpHb and LabHb) data points were recorded for final analysis, including 66 at which packed red blood cell transfusions were made. The accuracy of SpHb in comparison to LabHb was assessed using Bland-Altman analysis. The level of agreement between SpHb and LabHb for the 66 transfusion data points showed a 73% correlation (p < 0.001), bias of -0.313 g/dL with standard deviation of ± 1.06 g/dL, and limits of agreement of -2.44 g/dL and 1.81 g/dL. The level of agreement between SpHb and LabHb for all 137 data points showed a 72.7% correlation (p < 0.001), bias of -0.376 g/dL with standard deviation of ± 1.27 g/dL, and limits of agreement of -2.92 g/dL and 2.16 g/dL.
The researchers concluded that, “Continuous SpHb monitoring can aid us regarding early blood transfusion decisions in oncosurgical patients along with other measures such as clinical judgement by attending consultant and haemodynamic variables. It may improve the intraoperative management of oncosurgeries by helping in real time and continuous decision-making for blood transfusion.” They also noted that SpHb “allows the physician to focus on the haemoglobin trend and detect either a slow decrease or a significant rapid drop in haemoglobin and therefore decide the appropriate time to perform an invasive measurement of haemoglobin.”
As limitations, the researchers stated that they “collected venous blood sample from central venous line rather [than] arterial blood. Haemoglobin concentration has been reported to be higher in venous blood than arterial blood though precision for haemoglobin estimation is higher for venous blood.” In addition, they suggested that further research may be needed to assess the effect of colloid administration and skin temperature at the probe site on SpHb accuracy, as well as its accuracy on patients with blood loss rates differing from the “massive but steady” rates observed in this study.
SpHb monitoring is not intended to replace laboratory blood testing. Blood samples should be analyzed by laboratory instruments prior to clinical decision making.
References
1. Gupta N, Kulkami A, Bhargava AK, Prakash A, and Gupta N. Utility of noninvasive haemoglobin monitoring in oncosurgery patients. Indian Jour Anesth. July 2017; Volume 61; Issue 7; 543-548.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Patient SafetyNet, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Study Investigates Performance of Masimo PVi® As Part of Goal-Directed Fluid Therapy During Laparoscopic Bariatric Surgery
Neuchatel, Switzerland – August 21, 2017 – Masimo (NASDAQ: MASI) announced today the findings of a recently published study in which researchers at Firat University in Turkey evaluated the performance of Masimo PVi®, a noninvasive and continuous measurement of the dynamic changes in perfusion index (Pi) that occur during respiratory cycles, as the basis of a goal-directed fluid therapy (GDFT) protocol during laparoscopic bariatric surgery on mechanically-ventilated patients.1
In the study, Dr. Demirel and colleagues sought to evaluate whether using GDFT guided by PVi on morbidly obese patients undergoing laparoscopic Roux-en-Y gastric bypass (RYGB) surgery might result in less intravenous fluid use without compromising outcomes. They enrolled 60 patients and divided them randomly into control and GDFT groups. The control group's fluid levels were managed by standard fluid therapy, using mean arterial pressure (MAP) and central venous pressure (CVP) measured via a central venous access catheter as indicators of fluid responsiveness. The GDFT group's fluid status was monitored using a GDFT protocol based on PVi as a noninvasive, dynamic indicator of fluid responsiveness.
Both groups were initially administered 500 mL bolus colloid fluid at the beginning of surgery, followed by a continuous infusion of crystalloid fluid (4-8 mL/kg/h in the control group, or 2 mL/kg/h in the GDFT group per the protocol). In the control group, if CVP was less than 6 mmHg or MAP less than 65 mmHg, a 250 mL additional bolus of colloid fluid was administered. In the GDFT group, if PVi was greater than 14% for five minutes, the 250 mL colloid bolus was administered.
The researchers found that there was a significantly higher mean volume of crystalloid fluid administered in the control group (1499 mL ± 516.87 mL) compared to the GDFT group (1126 mL ± 234.98 mL) (p = 0.001). There were no significant differences in blood lactate levels (p greater than 0.05) or creatinine levels before and after surgery (p greater than 0.05) between the two groups.
The researchers concluded that, "Utilization of GDFT protocols based on PVi may prevent excessive intraoperative infusion of fluids in laparoscopic bariatric surgery. This method when intending to prevent intraoperative excessive fluid loading in RYGB surgery appears to have no effect on either renal functions or lactate levels. While this study shows the adequacy of PVi for fluid therapy in mechanically ventilated patients undergoing bariatric surgery, further research is warranted to assess adequacy of optimization of PVi."
Reference
1. Demirel I, Bolat E, Altun AY, Özdemir M, and Beştaş A. Efficacy of Goal-Directed Fluid Therapy via Pleth Variability Index During Laparoscopic Roux-en-Y Gastric Bypass Surgery in Morbidly Obese Patients. Obes Surg. 31 July 2017. DOI: 10.1007/s11695-017-2840-1.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo PVi®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo PVi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Dubai Health Authority Implements Masimo Patient SafetyNet™
Dubai, United Arab Emirates – August 14, 2017 – Masimo (NASDAQ: MASI) announced today that the Dubai Health Authority (DHA), the government organization that oversees the healthcare systems of Dubai, is augmenting its current inventory of Masimo equipment and technology with the implementation of Masimo Patient SafetyNet™*, a supplemental remote monitoring and clinician notification system, at two hospitals in Dubai.
Masimo Patient SafetyNet enables information from bedside monitors, such as Masimo Root® with the Radical-7® or wearable Radius-7® Pulse CO-Oximeter®, to be accessible from a central viewing station. When changes occur in measured values, which may indicate deterioration in a patient's condition, Patient SafetyNet automatically sends wireless alerts directly to clinicians, wherever they may be. In addition, Patient SafetyNet can automate the transfer of patient data, including admission data, vital signs, early warning scores (EWS), and other physiological parameters, directly to hospital electronic medical record (EMR) systems, helping to improve clinician workflows and reduce the possibility of transcription errors.
Dr. Andreas Taenzer and colleagues found in an 11-month study conducted at Dartmouth-Hitchcock Medical Center that using Patient SafetyNet and Masimo SET® pulse oximetry as part of a comprehensive alarm management strategy reduced rescue events by 65% and intensive care unit transfers by 48%, and as a result, reduced costs by $1,480,000.1,2 In a subsequent article, they announced that after five years, Dartmouth-Hitchcock had had zero preventable deaths or instances of brain damage due to opioids since the installation of Patient SafetyNet.2 In 2016, after ten years, they reported achieving a 50% reduction in unplanned ICU transfers and a 60% reduction in rescue events, despite increases in patient acuity and occupancy.3
The two Dubai Health Authority medical centers implementing Patient SafetyNet are Dubai Hospital (625 beds), which provides general medical and surgical care, and Latifa Hospital (367 beds), which specializes in maternal and child care. Dubai Hospital installed its first Patient SafetyNet in 2013. Latifa Hospital is in the process of installing four systems, with a further system planned for Dubai Hospital. "We are excited to deepen our partnership with Masimo," said Humaid Al Qatami, Chairman of the Board and Director General of Dubai Health Authority. "The Dubai Health Authority's mission is to develop an integrated and sustainable healthcare system that ensures our comprehensive services achieve the highest international standards, and we believe that Masimo's monitoring devices, now even more connected to hospital infrastructure through the power of Patient SafetyNet, will help us meet that goal."
"Patient SafetyNet, in conjunction with Masimo SET® pulse oximetry, enables continuous supplemental monitoring of active patients in post-surgical wards and can help save the lives of patients on opioids, among many other benefits," said Joe Kiani, Founder and CEO of Masimo. "We applaud the Dubai Health Authority, dedicated to providing no less than the best health care in the world, for recognizing the importance of implementing such a proven and powerful centralized monitoring and patient surveillance system."
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study. Anesthesiology. 2010 Feb;112(2):282-7.
2. Taenzer AH et al. Postoperative Monitoring - The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online.
3. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Patient SafetyNet, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Study Evaluates Performance of Masimo SpHb® in Trauma Patients
with Low Hemoglobin Levels
Neuchatel, Switzerland – August 7, 2017 – Masimo (NASDAQ: MASI) announced today the findings of a recently published study in which researchers at Cairo University in Egypt evaluated the performance of Masimo SpHb®, noninvasive, continuous hemoglobin measurement, in trauma patients with low hemoglobin levels.1
In the prospective, observational study, Dr. Gamal and colleagues sought to evaluate SpHb measurements on trauma patients with low hemoglobin levels (below 8 g/dL) because trauma patients are "vulnerable to frequent blood transfusion." They enrolled 70 adult patients with hemoglobin levels lower than 8 g/dL who were admitted to the emergency department (ED) of Cairo University Hospital and scheduled for surgical intervention. While in the ED, the patients’ SpHb was continuously monitored using a Masimo Radical-7® Pulse CO-Oximeter®, with initial baseline measurement recorded as well as measurement after each unit of blood was administered. The researchers simultaneously obtained 2 mL venous blood samples, which were analyzed using a Coulter LH 750 Beckman analyzer (LabHb).
A total of 184 samples with corresponding SpHb values were collected for final analysis. The distribution of LabHb values was 20 (11%) below 6 g/dL, 97 (53%) between 6-7 g/dL, and 67 (36%) between 7-8 g/dL. The accuracy of SpHb in comparison to LabHb was assessed using Bland-Altman analysis. The level of agreement between SpHb and LabHb showed a bias of 0.12 g/dL and limits of agreement of -0.56 g/dL and 0.79 g/dL.
To determine the accuracy of SpHb as a trend measurement, the researchers also observed the change in hemoglobin (DeltaHb) before and after each unit of blood was transfused, for both methods. The level of agreement between DeltaSpHb and DeltaLabHb showed a bias of -0.05 g/dL and limits of agreement of -0.62 g/dL and 0.51 g/dL.
The researchers concluded that "SpHb showed accurate precision in both absolute values and trend values compared to LabHb measurement in trauma patients with low hemoglobin levels." They also suggested several possible uses for SpHb, including as "a trend monitor that would alert the physician to any sudden bleeding mishaps," and as a "good supplementary measure" to LabHb that can "save time and effort."
The researchers acknowledged that this study is not sufficient to alone answer the question, "Can we transfuse blood relying solely on SpHb or not?" However, they noted that their findings add to the body of evidence in favor of SpHb and suggest the need for additional research regarding the role of Masimo technology in blood transfusion decisions.
SpHb monitoring is not intended to replace laboratory blood testing. Blood samples should be analyzed by laboratory instruments prior to clinical decision making.
The accuracy specification of SpHb is 1 g/dL ARMS* in the range of 8-17 g/dL. SpHb accuracy has been validated on healthy adult male and female volunteers and on surgical patients with light to dark skin pigmentation against an invasive laboratory device. SpHb accuracy has not been validated in conditions of motion or low perfusion.
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within ± ARMS of the reference measurements in a controlled study.
Reference
1. Gamal M, Abdelhamid B, Zakaria D, Abd El Dayem O, Ashraf R, Fawzy M, and Hasanin A. Evaluation of noninvasive hemoglobin monitoring in trauma patients with low hemoglobin levels. Shock. July 2017. DOI: 10.1097/SHK. 0000000000000949.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Study Compares Performance of Masimo Acoustic Respiration Rate (RRa®) and Nellcor Plethysmographic Respiration Rate in Volunteers
Tokyo, Japan – July 31, 2017 – Masimo (NASDAQ: MASI) announced today that researchers at the Tokyo Women's Medical University, Department of Anesthesiology, in Japan have published a study investigating the measurement of respiration rate in volunteers. Masimo acoustic respiration rate (Masimo), using the Masimo Radical-7® Pulse CO-Oximeter®, was studied alongside Nellcor plethysmographic respiration rate (Nellcor), using the Nellcor PM1000N.1
Dr. Kitsiripant and colleagues enrolled 50 healthy adult volunteers in the study. Respiration rate, pulse rate, and oxygen saturation (SpO2) values were measured using the two technologies: Nellcor respiration rate, pulse rate, and SpO2 on PM1000N (version 2.0.20.0) were measured using an SpO2 adhesive sensor on the left index finger; Masimo respiration rate on Radical-7 (V.7910, processor V1.3.06i) was measured using the RAS-125c (rev D) acoustic sensor on the left side of the neck, with an R1-25L adhesive sensor on the right index finger measuring Masimo pulse rate and SpO2.
-
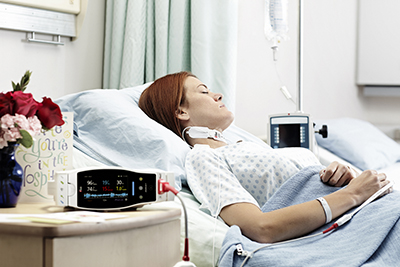
Masimo Radical-7® Pulse CO-Oximeter® with RAS-125c Respiratory Acoustic Sensor
Both devices were configured to alarm in the event of respiratory pause (Masimo) or low respiration rate (Nellcor) for 30 seconds, respiration rate under 10 breaths/minute, and SpO2 of 90% or below. The volunteers were required to breathe at a rate of 12 breaths/minute for 3 minutes, then hold their breath until one of the device’s respiratory pause/low respiration rate alarms was triggered, then resume breathing. Because of the difficulty for some volunteers of completely stopping airflow for 30 seconds, a smaller group of 10 volunteers was recruited to perform the same procedure but with respiratory pause/low respiration rate alarms set to 15 seconds.
Of the 143 procedures in which breathing was successfully held for more than 30 seconds, Masimo alarmed 114 times and Nellcor alarmed 15 times. The average time to alarm for Masimo was 35 seconds and for Nellcor, 59 seconds. Most of the alarms for Nellcor followed from SpO2 being less than 90%, whereas most for Masimo were caused by respiration rate less than 10 breaths/minute (which tended to occur prior to the drop in SpO2). Of the 29 procedures in which breathing was held for 15 seconds, Masimo alarmed 29 times, with an average time to alarm of 21 seconds, and Nellcor did not alarm at all.
The researchers concluded that Masimo acoustic respiration rate provided faster detection of respiratory pause than Nellcor, but it should be noted as a limitation of the study that the measurements were taken using volunteer participants who maintained a fixed breathing rate and then abruptly held their breath.
rainbow Acoustic Monitoring® sensors and cables are indicated for the continuous, noninvasive monitoring of respiratory rate (RRa®). The RAS-125c sensor is indicated for adult and pediatric patients, in hospitals, hospital-type facilities, mobile and home environments.
Reference
1. Kitsiripant C et al. Comparison of Nellcor™ PM1000N and Masimo Radical-7® for detecting apnea in volunteers. J Anesth. 9 July 2017. DOI: 10.1007/s00540-017-2385-4.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), and more recently, Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo PVi® and RPVi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo PVi and RPVi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces CE Marking of Noninvasive RPVi™, a rainbow® Multi-Wavelength Index of Pleth Variability
Neuchatel, Switzerland – July 20, 2017 – Masimo (NASDAQ: MASI) announced today the CE marking of RPVi™, a noninvasive and continuous measurement of the dynamic changes in perfusion index (Pi) that occur during one or more respiratory cycles. RPVi is designed to show changes that reflect physiologic factors such as vascular tone, circulating blood volume, and intrathoracic pressure excursions.
-
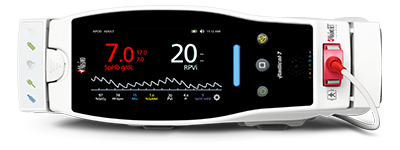
Masimo Radical-7® Pulse CO-Oximeter® with RPVi™
RPVi, powered by Masimo rainbow® technology, is a multi-wavelength version of the currently available Pleth Variability Index (PVi®). RPVi is designed to provide enhanced specificity to changes in fluid volume compared to PVi.1
Several peer-reviewed clinical studies have evaluated the utility of the currently available measurement PVi as an indicator of fluid responsiveness in mechanically-ventilated patients. For example:
- In a study of 82 patients undergoing major abdominal surgery, researchers found that PVi-based goal-directed fluid management reduced the volume of intraoperative fluid infused and reduced intraoperative and postoperative lactate levels.2
- In a study of 109 patients undergoing colorectal surgery, researchers found that the implementation of an enhanced recovery protocol which included PVi led to improved patient satisfaction and substantial reduction in lengths of stay, complication rates, and costs for patients undergoing both open and laparoscopic colorectal surgery.3
Joe Kiani, Founder and CEO of Masimo, said, "Masimo rainbow® technology, first announced in 2005, continues to drive innovative new measurements and improvements to existing ones. We are proud to introduce RPVi, and hope that it will be useful to clinicians around the world in helping to improve patient care."
RPVi is not available for sale in the United States.
References
1. Masimo data on file.
2. Forget P et al. Goal-Directed Fluid Management Based on the Pulse Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management. Anesth Analg. 2010; 111(4):910-4.
3. Thiele RH et al. Standardization of Care: Impact of an Enhanced Recovery Protocol on Length of Stay, Complications, and Direct Costs After Colorectal Surgery. Journal of the American College of Surgeons. 2015. Doi: 10.1016/j.jamcollsurg.2014.12.042.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo PVi® and RPVi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo PVi and RPVi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces CE Marking of rainbow® Super Sensor
Masimo Super Sensor is the First Ever to Measure Arterial Oxygen Saturation (SpO2), Hemoglobin (SpHb®), Carboxyhemoglobin (SpCO®), Methemoglobin (SpMet®), Pleth Variability Index (PVi®), Index of Perfusion (Pi), and Pulse Rate (PR), All Noninvasively
Neuchatel, Switzerland – July 10, 2017 – Masimo (NASDAQ: MASI) announced today the CE marking of the rainbow® Super DCI®-mini sensor, a reusable spot-check sensor that features Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry and rainbow SET™ technology with multiple physiologic measurements – including, for the first time, the ability to measure total hemoglobin (SpHb®), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and arterial oxygen saturation (SpO2) using the same noninvasive reusable sensor.
In 2016, Masimo introduced the rainbow® DCI-mini sensor, enabling spot-check measurement of Next Generation SpHb and other parameters. Now, with the rainbow® Super DCI-mini sensor, an expanded set of parameters can be measured using a single sensor: SpO2, pulse rate (PR), perfusion index (Pi), pleth variability index (PVi®), SpHb, SpCO, and SpMet.
-
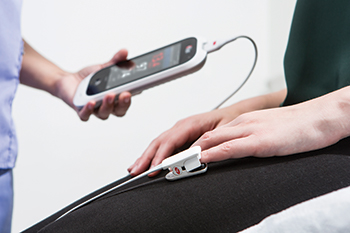
Masimo Rad-67™ Pulse CO-Oximeter® with rainbow® Super DCI®-mini Sensor
The rainbow® Super DCI-mini sensor can be used to spot-check all patients weighing 3 kg or more, further reducing the need for multiple sensor types; the sensor can be applied to an adult finger, a pediatric finger, or an infant finger, thumb, or great toe. The sensor is small and lightweight, with a flexible cable to provide sensor stability and patient comfort during monitoring.
Next Generation SpHb technology offers motion tolerance and a faster time to display SpHb results (in as few as 30 seconds). In addition, field performance has been enhanced in lower hemoglobin ranges. Next Generation SpHb is enabled when the Rad-67™ Pulse CO-Oximeter® and the DCI-mini or Super DCI-mini sensor are used together.
SpCO monitoring may lead to the identification of elevated carbon monoxide levels that might otherwise go undetected in front-line settings, such as triage and emergency care. SpMet helps clinicians monitor for methemoglobin in care areas where the drugs that cause methemoglobinemia are most common, such as procedure labs and the operating room.
Joe Kiani, Founder and CEO of Masimo, said, "This is an exciting day for us and hopefully a great opportunity to improve patient care. Since the invention of rainbow® technology, we have been wanting our customers to be able to measure SpCO, SpMet, SpHb and SpO2 simultaneously. Now they can! In addition, we have been pursuing a spot-check sensor that fits all patients across the age spectrum. We believe the rainbow® Super DCI-mini sensor will be especially valuable for use in triage and emergency care situations. We plan to introduce a continuous measurement version of the Super Sensor in the near future."
The rainbow® Super DCI-mini sensor, Next Generation SpHb, and Rad-67 have not received FDA 510(k) clearance and are not available for sale in the United States.
SpCO is intended to be used to monitor CO levels in the blood and is not intended to be used as the sole basis for making diagnosis or treatment decisions related to carbon monoxide poisoning. SpMet is not to be used as the sole basis for making diagnosis or treatment decisions related to methemoglobinemia. SpHb, SpCO, and SpMet monitoring are not intended to replace laboratory blood testing; blood samples should be analyzed by laboratory instruments prior to clinical decision making.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo rainbow® Super DCI®-mini sensor. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo rainbow® Super DCI-mini sensor, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com


New Study Compares Performance of Masimo Next Generation SedLine® Patient State Index (PSi) to Original PSi During Anesthesia
Geneva, Switzerland – June 19, 2017 – Masimo (NASDAQ: MASI) announced today the findings of an abstract presented at Euroanaesthesia 2017 in Geneva, Switzerland. In the study, researchers at University Medical Center Groningen, Netherlands compared original and Next Generation versions of Masimo Patient State Index (PSi, a processed EEG parameter related to the effect of anesthetic agents) during Masimo SedLine® brain function monitoring of patients under propofol and sevoflurane anesthesia.1
-
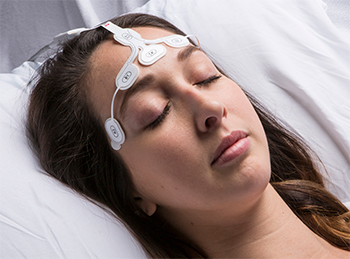
Masimo RD SedLine® Adult EEG Sensor
SedLine brain function monitoring features four simultaneous EEG leads to enable continuous assessment of both sides of the brain, four EEG waveforms, a Density Spectral Array (DSA; an easy-to-interpret, high-resolution display of bi-hemispheric activity and EEG power), and PSi. Next Generation SedLine enhances PSi to make it less susceptible to electromyographic (EMG) interference and to improve its performance in low-power EEG cases.
In the study, Dr. Kuizenga and colleagues sought to compare the original and Next Generation PSi algorithms, referred to as PSi-1 and PSi-2, respectively, as they correlated with propofol and sevoflurane drug concentrations and with the Modified Observers Assessment of Alertness and Sedation (MOAAS) scale. They also sought to assess the influence of 2 and 4 ng/mL effect-site concentrations of remifentanil on the performance of the two algorithms.
The researchers enrolled 36 healthy volunteers, stratified by age, and assigned them randomly to a sequence of four sessions of anesthesia. In one session, propofol was administered in a series of graded steps; in the second, sevoflurane was similarly administered; in the third and fourth, the two concentrations of remifentanil were also administered. During each step of each session, after a twelve-minute delay for equilibration, MOAAS was tested and a blood sample was taken to measure drug concentrations. EEG was collected using Masimo Root® with SedLine, from which time-synchronized PSi-1 and PSi-2 values were later extracted. The researchers then plotted MOAAS, drug concentration, PSi-1, and PSi-2 values over time.
The researchers found that when charted against drug concentrations, PSi-2 showed "reduced population variability and improved baseline stability" compared to PSi-1. When charted against MOAAS, PSi-2 had "lower interindividual variability" than PSi-1. They also noted that "Both PSis distinguish MOAAS 5, 4, and 3 better during propofol anesthesia compared to sevoflurane. This difference disappears when adding remifentanil."
The investigators concluded that "PSi-2 [Next Generation SedLine PSi] has enhanced signal stability and a better description of the dose-response relationship. PSi-2 has therefore improved capacity as a pharmacodynamic monitor of anesthesia compared to PSi-1."
Next Generation SedLine has not received 510(k) clearance and is not available for sale in the United States.
Reference
1. Kuizenga M.H., Colin P.J., Vereecke H.E.M., Struys M.M.R.F. Comparison between two versions of the Patient State Index during propofol and sevoflurane anesthesia, with or without remifentanil. Proceedings from Euroanaesthesia 2017, Geneva, Switzerland. Abstract #01AP07-4.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Investigates the Clinical Utility of ORi™, Masimo Oxygen Reserve Index™
Neuchatel, Switzerland – June 12, 2017 – Masimo (NASDAQ: MASI) announced today the findings of an abstract presented at the recent International Anesthesia Research Society (IARS) Annual Meeting in Washington, DC. In the study, researchers at the UC Davis School of Medicine evaluated the potential clinical utility of Masimo Oxygen Reserve Index™ (ORi™) as an early warning of arterial hemoglobin desaturation in critically ill patients.1
ORi is a relative indicator of the partial pressure of oxygen in arterial blood (PaO2) in the range of 100 to 200 mmHg. ORi is intended to supplement, not replace, oxygen saturation (SpO2) monitoring and PaO2 measurements. As an "index" parameter with a unit-less scale between 0 and 1, ORi can be trended and has optional alarms to notify clinicians of changes in a patient's oxygen reserve.
In the prospective, collaborative, observational study, Dr. Leonard Lee and colleagues enrolled 40 adult critically ill patients who were scheduled for elective surgical procedures requiring endotracheal intubation and planned arterial pressure monitoring catheter placement prior to induction of general anesthesia. The patients' ORi values were measured using a Masimo Radical-7® Pulse CO-Oximeter®. The researchers recorded the time elapsed from the start of ORi alarming (triggered by decrease in the absolute value and rate of change in ORi) to 94% oxygen saturation, as well as the time elapsed from 98% to 94% saturation. The average time interval between the start of ORi alarming and 98% saturation was considered to be the average increase in warning time provided by ORi.
The researchers found that among the patients, the average time from the start of ORi alarming to 94% oxygen saturation was 80±38 seconds (ranging from 29 to 227 seconds). The average time from 98% to 94% saturation was 46±23 seconds (ranging from 12 to 108) seconds. Therefore, the average increase in warning time provided by ORi was 34±23 seconds (ranging from 4 to 119) seconds. On a percentage basis, the increase provided by ORi was 96%±92% (ranging from 5% to 479%).
The researchers concluded that the study "demonstrates the potential utility of ORi as an advanced warning of arterial desaturation and as an adjunct to SpO2. This additional warning time can potentially translate to improved patient safety by allowing earlier calls for help, assistance from a more experienced person, or modification of airway management. For this analysis we defined the advance warning to end at 98% SpO2. In clinical situations this SpO2 might not be considered to be critical. Using a lower SpO2 as the alarm level would increase the advance warning provided by ORi. Further analysis of the correlation of ORi and PaO2, the use of ORi as a guide to pre-oxygenation, and its utility in the morbidly obese are areas for future study."
In another recent study, researchers at Children's Medical Center in Dallas, Texas concluded that ORi could provide clinicians with a median of 31.5 seconds advanced warning of impending desaturation in pediatric patients with induced apnea after pre-oxygenation.2
ORi has not received 510(k) clearance and is not available for sale in the United States.
References
1. Lee L, Singh A, Applegate R, and Fleming N. Oxygen Reserve Index: An early warning for desaturation in critically ill patients. Proceedings from the 2017 IARS Annual Meeting, Washington, DC. Abstract #A1406.
2. Szmuk P et al. Oxygen Reserve Index A Novel Noninvasive Measure of Oxygen Reserve – A Pilot Study. Anesthesiology. 4 2016, Vol. 124, 779-784. doi:10.1097/ALN.0000000000001009.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo ORi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo ORi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces Limited Market Release of Rad-67™ rainbow® Pulse CO-Oximeter® with Next Generation SpHb®
-
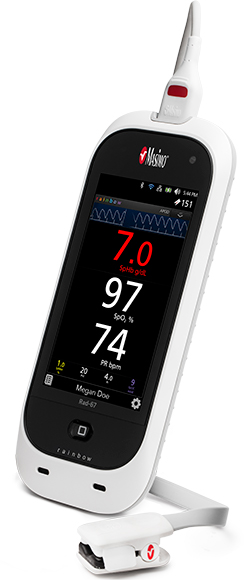
Masimo Rad-67™ Handheld Pulse CO-Oximeter® with rainbow® DCI®-mini Sensor
Neuchatel, Switzerland – June 5, 2017 – Masimo (NASDAQ: MASI) announced today, in conjunction with its CE marking, the limited market release of the Spot-Check Rad-67™ Handheld Pulse CO-Oximeter®. Rad-67 offers Measure-through Motion and Low Perfusion™ SET® pulse oximetry and upgradeable rainbow® noninvasive monitoring technology in a compact, portable spot-check device. With the universal reusable rainbow® DCI®-mini sensor, Rad-67 features Next Generation SpHb® (noninvasive total hemoglobin) technology.
Next Generation SpHb technology offers improved motion tolerance and a faster time to display SpHb results (in as few as 30 seconds). In addition, field performance has been enhanced in lower hemoglobin ranges. The rainbow® DCI-mini sensor allows patients of all ages, from infants to adults, to be spot-checked using a reusable sensor. Next Generation SpHb, enabled when Rad-67 and the DCI-mini sensor are used together, significantly advances the forefront of noninvasive portable hemoglobin spot-checking.
Rad-67's ability to provide portable spot-check measurements of both oxygen saturation and SpHb, in addition to other noninvasive rainbow® measurements, makes it a useful single-device solution in multiple care areas and mobile environments, such as when screening patients during blood donation drives, emergency room screening, and in physicians' offices. In addition to a rechargeable battery with six-hour run time, Rad-67 features a high-resolution, HD color display with intuitive touchscreen navigation that automatically adjusts brightness to optimize visibility in a variety of settings, including outdoors. The slim-profile sensor connector port is designed to provide tactile feedback upon proper connection. Multiple parameters are simultaneously displayed, allowing for quick patient assessment.
Rad-67 provides convenient historical data review directly on the device, with unique patient identifiers to help improve organization of records and workflow. Using Bluetooth®, Rad-67 can connect wirelessly to portable printers. Rad-67 also includes built-in wireless connectivity via Wi-Fi. When used in conjunction with Masimo Iris Gateway™ or Patient SafetyNet™*, data from Rad-67 can be sent directly to the patient's electronic medical record (EMR). Automating data transfer with EMR integration may help reduce the possibility of human error when manually transferring data.
Dr. Aryeh Shander, Chief of Anesthesiology, Critical Care Medicine at Englewood Hospital and Medical Center, noted that, "in the process of screening for anemia, having the ability to detect hemoglobin noninvasively is one of the greatest advances. Once low hemoglobin is reported, confirmation with a conventional laboratory test will further explore the possible causes."
Joe Kiani, Founder and CEO of Masimo, said, "We're proud to announce Rad-67 with Next Generation SpHb technology. Next Generation SpHb represents a significant enhancement to the noninvasive measurement we invented a decade ago – a measurement we look forward to continuing to improve."
Rad-67 has not received 510(k) clearance and is not available for sale in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 including 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Rad-67™ Pulse CO-Oximeter®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-67 Pulse CO-Oximeter, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo and Mdoloris Announce Masimo Open Connect™ (MOC) Partnership for the Root® Patient Monitoring and Connectivity Hub
Analgesia Nociception Index (ANI®) Module for Root to be Previewed at the European Society of Anaesthesiology in Geneva, Switzerland June 3 – 5, 2017
Irvine, California and Lille, France – June 1, 2017 – Masimo (NASDAQ: MASI) and Mdoloris Medical Systems announced today a Masimo Open Connect™ (MOC) partnership for the Root® Open Architecture Patient Monitoring and Connectivity Hub, in which Mdoloris is developing and will commercialize their Analgesia Nociception Index (ANI®) technology for Root.
Masimo's unique approach to medical technology integration through MOC partnerships is expected to address some major barriers to new technology adoption in patient monitoring. Root's open architecture and built-in connectivity enable third-party companies to determine themselves whether to pursue an integrated product. They can then independently develop, obtain regulatory approvals, and commercialize their own external MOC-9 module or Masimo Open Connect Control (MOC-C™) App for Root using Masimo's MOC software development kit (SDK). Masimo’s engineering team supports MOC partner development as needed and Masimo's commercial team helps increase awareness of the availability of MOC-9 modules and MOC-C Apps from MOC partners. In turn, MOC partners use their existing distribution channels to sell their MOC-9 module or MOC-C App to Masimo customers already using Root, as well as offering the MOC-9 module or MOC-C App for Root to their potential customers as an additional option to implement their technology.
Pain relief is a major concern of all developed countries while pain assessment has historically been subjective, time-consuming due to its manual nature, and intermittent. To address these limitations, Mdoloris has developed ANI technology to provide an objective, noninvasive, and continuous way to automatically monitor the pain level of patients. Use of the ANI may help clinicians improve pain assessment and through it, patient care and pain management with more objective and precise drug administration.
"We are proud to announce our first MOC partnership and expect Root’s advanced patient monitoring and versatile connectivity options will be strengthened through MOC development and the addition of innovative new technologies such as ANI from Mdoloris," said Joe Kiani, Founder and CEO of Masimo. "We believe that Root with Masimo Open Connect can do for patient monitoring what the PC did for computing: speed up the patient monitoring innovation cycle, reduce the cost of patient monitoring, and prolong the useful life of the equipment hospitals invest in."
"We consider Masimo to be the most innovative company in the patient monitoring space, so it is a pleasure to announce our MOC partnership. We share Masimo’s vision of improving patient outcomes and reducing the cost of care and believe Root offers a unique and compelling solution to implement our ANI technology," said Fabien Pagniez, Founder and CEO of Mdoloris Medical Systems.
The ANI MOC-9 module for Root will be available for preview in the Mdoloris exhibit booth at the European Society of Anaesthesiology in Geneva, Switzerland, June 3 to 5, 2017.
ANI has not received 510(k) clearance and is not available for sale in the United States.
About Mdoloris Medical Systems
Mdoloris (www.mdoloris.com), a medical devices manufacturer, was created in June 2010 out of 23 years of academic research performed in Lille University hospital, France. It has an international representation in more than 58 countries and a scientific, technical and medical acknowledgement. Mdoloris has developed so far three products, all able to continuously assess the pain level of patients (The ANI technology for patients older than two years old, the NIPE technology for neonates, the PTA technology for pets). Its innovative technologies provide clinical added values for clinicians that are not able to communicate with their patients in order to personalize pain medications and avoid known side effects due to over / under dosage of such drugs.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 including 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
ORi has not received 510(k) clearance and is not available for sale in the United States.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Open Connect™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Open Connect, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Antoine Szczypa
Mdoloris
Email: Antoine.szczypa@mdoloris.com
Potential MOC Partners
Visit: https://professional.masimo.com/products/continuous/moc9/
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces FDA 510(k) Clearance of O3™ Regional Oximetry for Pediatric Patients
-

Masimo O3™ Regional Oximetry Pediatric (top) and Adult (bottom) Sensors
Irvine, California – May 30, 2017 – Masimo (NASDAQ: MASI) announced today FDA 510(k) clearance for the pediatric indication for O3™ regional oximetry with the O3 pediatric sensor. Regional oximetry, also referred to as tissue or cerebral oximetry, may help clinicians monitor cerebral oxygenation in situations in which peripheral pulse oximetry alone may not be fully indicative of the oxygen in the brain. With the clearance of the O3 pediatric sensor, O3 regional oximetry monitoring, which was already available for adult patients in the United States, is now also available for pediatric patients weighing more than 5 kg (11 lbs) and less than 40 kg (88 lbs).
O3 regional oximetry uses near-infrared spectroscopy (NIRS) to continuously monitor absolute and trended regional tissue oxygen saturation (rSO2) in the cerebral region. Early detection and correction of imbalances in oxygen delivery to the brain are important tools in helping patients avoid postoperative morbidity and adverse outcomes.1
"O3 regional oximetry provides access to valuable data about cerebral oxygen saturation," said Joe Kiani, Founder and CEO of Masimo. "With adult and pediatric trend accuracy of 3% and absolute accuracy of 4% and 5% on adults and pediatrics respectively,2 without controlling CO2, Masimo O3 should help clinicians build a better picture of brain oxygenation – and hopefully better outcomes for all of their patients, including pediatrics as young as three-months old."2
In addition, Masimo O3 regional oximetry and SedLine® brain function monitoring are both available on a single platform, Masimo Root® – opening up a path to better understanding of the brain.
References
1. Booth, Dukatz, Ausman, and Wider. Cerebral and somatic venous oximetry in adults and infants. Surg. Neurol Int. 2010; 1: 75.
2. Masimo data on file.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 including 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo O3™ regional oximetry. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo O3 regional oximetry, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces the Full Market Release of Early Warning Score (EWS) with the Root® Patient Monitoring and Connectivity Hub
Irvine, California – May 19, 2017 – Masimo (NASDAQ: MASI) announced today the full market release of Early Warning Score (EWS) on the Root® patient monitoring and connectivity hub. EWS aggregates information from multiple vital signs and clinical observations to generate a score that represents the potential degree of patient deterioration.
Root, which works in conjunction with Radical-7® or Radius-7® Pulse CO-Oximeters® and Masimo Open Connect™ (MOC-9™) measurements, features Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, rainbow SET™ pulse CO-Oximetry, NomoLine™ capnography and gas monitoring, SedLine® brain function monitoring, O3™ regional oximetry, and SunTech® blood pressure and Welch Allyn® temperature monitoring. Masimo SET® helps clinicians monitor oxygen saturation and pulse rate during motion and low perfusion for more than 100 million patients a year1, including at 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.2
-
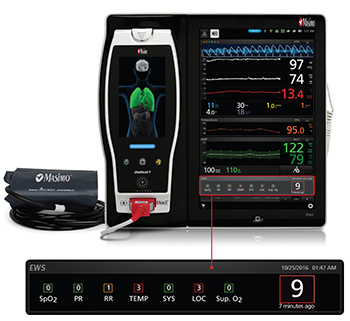
Masimo Root® with Early Warning Score (EWS)
Patient data from Radical-7 or Radius-7 and data collected using Root and other connected Masimo and third-party devices can be shared with Masimo Patient SafetyNet™*, providing hospital-wide remote monitoring and clinician notification, as well as the ability to automatically transfer patient data to a hospital's Electronic Medical Record (EMR). Each time a clinician transfers data to the EMR via Root connected to Patient SafetyNet, an Early Warning Score (EWS) can now be included. Clinicians can also choose to have the standalone Root, not connected to Patient SafetyNet, perform EWS calculations, helping assist spot-check-based nursing workflows.
Early warning scores are based on multiple contributors, including vital signs such as oxygen saturation, pulse rate, respiration rate, body temperature, and systolic blood pressure – and contributors entered by clinicians, such as level of consciousness, use of supplemental oxygen, and urine output. The weighting and number of contributors differ depending upon which EWS protocol is used. Root can be customized for various predefined EWS protocols, or hospitals can configure their own set of required contributors, and their relative weights, to create an EWS unique to their care environment.
"Root, from its versatile connectivity options to its advanced patient monitoring, from rainbow® SpHb® to SET® SpO2, helps hospitals improve patient care. We hope the addition of Early Warning Score to Root will help automate a potentially valuable calculation and streamline its inclusion in EMRs," said Joe Kiani, Founder and CEO of Masimo.
EWS is a convenient aid to clinical assessment and not a substitute for clinical judgment.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Estimate: Masimo data on file.
2. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 including 16 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.



Midmark, Masimo Announce Partnership to Improve Vital Signs Acquisition
DAYTON, Ohio, May 15, 2017 – Midmark Corporation, a leading medical solutions provider for ambulatory care, and Masimo (NASDAQ: MASI), a global leader in innovative noninvasive monitoring technologies, today announced a partnership focused on improving the accuracy and efficiency of vital signs acquisition in the clinical space.
With this partnership, Masimo SET® pulse oximetry technology is now an available option with Midmark’s recently introduced Midmark IQvitals® Zone™, the industry’s first monitoring device to feature Bluetooth® low energy technology for auto-connecting during the vitals acquisition process. IQvitals Zone devices equipped with Masimo SET® technology provide clinicians with accurate, real-time vitals sign information including oxygen saturation (SpO2), pulse rate (PR), and perfusion index (Pi) to facilitate timely clinical decisions.
Midmark IQvitals Zone changes the traditional workflow in vitals acquisition by establishing a direct wireless connection between vital signs measurement and the caregiver’s tablet or laptop. The vital signs monitor automatically connects with the appropriate equipment when the caregiver places a tablet or laptop on the multi-use work surface or near the monitoring device and initiates vitals acquisition. When the equipment is connected, caregivers can take patient vitals, review results and seamlessly import the information to an electronic medical records (EMR) system directly from their laptop or tablet.
“Masimo has long been a leader in creating new technologies to improve patient care,” said Kurt Forsthoefel, Marketing Director, Medical Products and Services, Midmark. “Midmark and Masimo are two companies ultimately focused on improving outcomes and the healthcare experience, and we’re looking forward to working together to provide caregivers with innovative technologies that enhance patient-caregiver interaction and improve outcomes at the point of care.”
“Like Midmark, Masimo is focused on developing solutions which can help caregivers enhance the quality and process of care,” said Rick Fishel, President of Worldwide OEM Business and Strategic Development for Masimo. “We are confident that Masimo’s proven portfolio of accurate and timely measurements, in combination with Midmark’s innovative solutions, will help physicians and caregivers deliver quality care and enhance clinical work flow.”
The Midmark IQvitals Zone device with Masimo SET pulse oximetry technology is one part of a fully connected point of care ecosystem that promotes well-coordinated patient experiences. More information about how IQvitals Zone brings the industry one step closer to a connected ecosystem can be found in the new white paper, “Zone Technology: Connecting Vitals Acquisition within the Point of Care Ecosystem” in the Midmark content and news library.
The study was supported by research grants from Masimo to Loma Linda University, Mayo Clinic in Florida, and the University of California, Irvine.
About Midmark
Midmark Corporation, a privately held company founded in 1915, is a leading provider of medical, dental and veterinary equipment and technologies. Our nearly 1,700 teammates worldwide are passionate, courageous leaders focused on making a positive difference in the lives we touch by improving the experience between patients and their caregivers. Headquartered in Dayton, Ohio, Midmark maintains production and administrative offices in Versailles, Ohio, as well as seven other locations in the United States and international subsidiaries in India, Italy and the United Kingdom. To learn more about Midmark, visit midmark.com.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 including 17 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Contacts:
Meghann Naveau
Content & Social Media Manager
Midmark Corporation
Phone: (937) 281-7609
Email: mnaveau@midmark.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Multicenter Study at UC Davis, UC Los Angeles, and Mayo Clinic Evaluates the Trend Accuracy of Masimo Continuous SpHb®
Washington, DC – May 15, 2017 – Masimo (NASDAQ: MASI) announced today the findings of an abstract presented at the International Anesthesia Research Society (IARS) Annual Meeting in Washington, DC. In the multicenter study, researchers at three academic medical centers evaluated the trend accuracy of noninvasive, continuous hemoglobin monitoring (SpHb®).1
The prospective observational study was a collaboration among Drs. Richard Applegate and Patricia Applegate of the University of California, Davis; Dr. Maxime Cannesson of the University of California, Los Angeles; and Drs. Prith Peiris, Beth Ladlie, and Klaus Torp of the Mayo Clinic in Jacksonville, Florida.
The researchers enrolled 135 adult patients who were scheduled for surgery with planned arterial catheter placement and continuous SpHb monitoring. Each time a blood sample was obtained, the researchers recorded SpHb using a Masimo Radical-7® Pulse CO-Oximeter® with Masimo rainbow® disposable R125 sensors (Revision K; same algorithm as Revision L). They also analyzed each blood sample twice to determine clinical laboratory hemoglobin (tHb; Sysmex 550 or Coulter LH 750), arterial blood gas CO-oximeter hemoglobin (ABGHb; Radiometer ABL800, Nova Biomedical CCX or PhOX, or Siemens RAPIDLab 1265), and point-of-care hemoglobin (aHQHb; HemoCue HB 301).
To assess overall trend accuracy and trend accuracy within defined ranges, the researchers analyzed the correlation of change in tHb to changes in SpHb, ABGHb, and aHQHb. Trend bias within 10% of tHb was considered clinically equivalent. The researchers found that "The confidence intervals for the proportion of samples with trend bias within 10% of tHb overlapped for SpHb (372 of 416 trends; 89.4%; 86.1% to 92.2%), ABGHb (391 of 416 trends; 94.0%; 91.3 to 95.9%) and aHQHb (406 of 416 trends; 97.6%; 95.6 to 98.7%)."
The researchers concluded that "SpHb, ABGHb and aHQHb appear to provide similar intraoperative guidance regarding tHb increase or decrease. Continuous noninvasive SpHb changes larger than ± 0.5 g/dL could provide a reasonable indication for the clinician to obtain a confirmatory blood sample for Hb measurement, but not replace such measurement in guiding transfusion decision making. The transfusion impact of continuous hemoglobin trend monitoring should be studied."
SpHb monitoring may provide additional insight to the directional trend of hemoglobin between invasive blood samplings – when the SpHb trend is stable and the clinician may otherwise think hemoglobin is decreasing; when the SpHb trend is rising and the clinician may otherwise think hemoglobin is not rising fast enough; or when the SpHb trend is decreasing and the clinician may otherwise think hemoglobin is stable. Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
The study was supported by research grants from Masimo to Loma Linda University, Mayo Clinic in Florida, and the University of California, Irvine.
Reference
1. Applegate R, Cannesson M, Applegate P, Peiris P, Ladlie B, and Torp K. Hemoglobin Change Measurement Accuracy Obtained from 3 Devices During Surgery. Proceedings from the 2017 IARS Annual Meeting, Washington, DC. Abstract #A1786.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 including 17 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Introduces Rad-G™ Pulse Oximeter
Irvine, California – May 9, 2017 – Masimo (NASDAQ: MASI) today announced the introduction of Rad-G™, a combined pulse oximeter designed primarily for use in pneumonia screening and spot-checking of oxygen saturation (SpO2) in low-resource settings. The development of the device is supported in part by a grant of $4.95 million from the Bill and Melinda Gates Foundation (BMGF), announced in November 2016, as part of a partnership with Masimo to facilitate screening for pneumonia by health workers in low-resource areas.
-
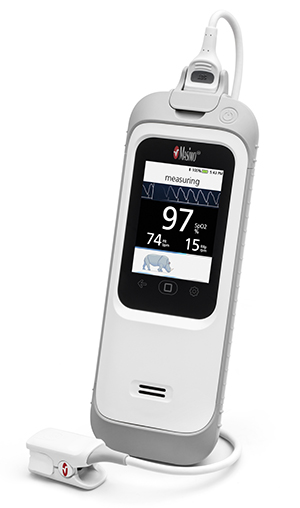
Masimo Rad-G™ Pulse Oximeter
Rad-G is a low-cost, rugged, handheld pulse oximetry device with a rechargeable battery and LCD display. It uses Masimo Measure-through Motion and Low Perfusion™ SET® pulse oximetry technology to measure SpO2, respiration rate from the Pleth (RRp™), pulse rate (PR), and perfusion index (Pi).
Pneumonia remains the single largest treatable infectious cause of death in children worldwide, causing over 900,000 deaths each year among children under 5 years of age.1 In a study funded by the BMGF Diagnostics Modelling Consortium, the researchers concluded that in settings where supplemental oxygen is available, the addition of pulse oximetry to standard integrated management of child illness protocols could reduce pneumonia mortality rates.2 More recently, the World Health Organization (WHO) has been conducting a multi-country evaluation of enhanced community case management of pneumonia with the use of Masimo SET® pulse oximetry by community health workers.3
Enhancing patient screening is critically important to reducing the global burden of pneumonia. Moreover, enhanced screening may empower healthcare providers by supporting informed decisions related to pneumonia diagnosis and treatment, with the appropriate administration of antibiotics and oxygen therapy when needed. Masimo and BMGF hope that Masimo pulse oximetry technology, already used to monitor approximately 100 million patients around the world, can help better screen children in even the most challenging conditions.
Joe Kiani, Founder and CEO of Masimo, said, "The introduction of the Rad-G is a critical milestone in our partnership with the Bill and Melinda Gates Foundation to help improve pneumonia screening. We are grateful to have the opportunity to bring our proven SET® pulse oximetry technology to areas of the world that are in desperate need of better healthcare, and look forward to making a positive difference in the lives of many children."
Rad-G is currently not available for sale in the United States, Canada, or the E.U.
References
1. Pneumonia Fact Sheet, World Health Organization (WHO), September 2016. http://www.who.int/mediacentre/factsheefts/fs331/en/.
2. Floyd J et al. Evaluating the impact of pulse oximetry on childhood pneumonia mortality in resource-poor settings. Nature. 2015 Dec 3;528(7580):S53-9.
3. World Health Organization (WHO), 2016.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 including 17 of the top 20 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Rad-G™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-G, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Two Fire Rescue Departments Honored for Excellence in Emergency Medical Services
Fire Service-Based EMS Awards Presented at 29th Annual National Fire and Emergency Services Dinner
Washington DC and Irvine, California – April 11, 2017 – Masimo (NASDAQ: MASI) announced today that the Congressional Fire Services Institute (CFSI) has honored the Orange County (Florida) Fire Rescue Department and the Montgomery County (Maryland) Fire & Rescue Service with the Excellence in Fire Service-Based EMS Award for best practices and innovative solutions in the delivery of emergency medical services. The awards presentation took place on April 6th in Washington, DC. Masimo is proud to be a co-sponsor of this award.
Orange County (Florida) Fire Rescue Department
The Orange County Fire Rescue Department was awarded the Excellence in Fire Service-Based EMS Award in recognition of the development of two best practices. The first is the Sepsis Alert Program, which educates EMS providers to better identify potential cases of severe sepsis among patients in pre-hospital settings, as early recognition and treatment may improve patient outcomes and lessen the financial impact of prolonged hospital stays. The program is modeled after similar “alert” programs that trigger EMS responders to initiate certain procedures, such as programs designed to recognize signs of stroke and cardiac arrest. Preliminary results since establishing the Sepsis Alert Program have included reductions in time to blood culture, time to administer antibiotics, time to administer fluids, length of hospital stay, and percentage admitted to the ICU.
The other best practice, the Paramedic Preceptor Academy, was developed to increase pass rates for Orange County’s new paramedics, as well as to improve training and continuing education opportunities identified through the department’s quality assurance process. As a result of the improved and standardized training, in the first year of the program first-time pass rates on written and practical assessments have increased dramatically. Several other central Florida fire departments are now also participating in the Paramedic Preceptor Academy, in the hopes of seeing similar results.
Montgomery County (Maryland) Fire & Rescue Service
The Montgomery County Fire & Rescue Service was awarded the Excellence in Fire Service-Based EMS Award in recognition of the creation of an outreach initiative called Montgomery County Non-Emergency Invention and Community Care Coordination (MCNIC3). Montgomery County developed MCNIC3 to address the disparity between the volume of emergency 911 calls, which continues to grow, and EMS resources, which have stayed relatively flat. Much of the growth in 911 calls is a result of low-priority calls from frequent 911 users. MCNIC3 targets these frequent 911 users and attempts to connect them with a variety of community-based medical and social programs, in an effort to better meet their ongoing healthcare needs without taking emergency care away from those with true emergency needs. Firefighters and EMS providers are able to refer any patient they encounter to the MCNIC3 program. By partnering with local hospitals, the health department, and various community groups, MCNIC3 can arrange periodic in-person visits to ensure that the patients’ healthcare needs are being met without straining county resources. To date, MCNIC3 has reduced calls for emergency service from those patients enrolled in the program by 55%. There are plans to expand the scope of the program and develop additional community partnerships.
“CFSI takes great pride in co-sponsoring the Excellence in Fire Service-Based EMS Awards with Masimo,” said Dr. William F. Jenaway, Ph.D., President of CFSI. “Through this awards program, we are able to recognize innovations in the delivery of emergency medical services throughout the nation. The recipients of the 2017 award, Orange and Montgomery Counties, certainly demonstrated themselves worthy of this recognition by developing outstanding new programs that improve training, distribute resources more wisely, and ultimately improve patient outcomes. We hope other departments around the country will be inspired to introduce similar innovations.”
“Firefighters and emergency services personnel are our heroes. In saving lives, they put their own on the line for us every day. It’s crucial they have the training and resources they need to provide the best care for those they protect. Recognizing the possibility of and intervening to address sepsis as soon as possible, for example, can significantly improve patient outcomes,” said Joe Kiani, Founder and CEO of Masimo. “Masimo is honored to co-sponsor this awards program with CFSI and to recognize Orange and Montgomery Counties for their innovative achievements.”
Approximately 1,600 fire and emergency services leaders attended the 29th Annual National Fire and Emergency Services Dinner to pay tribute to the dedication and commitment of the nation's fire and emergency services providers. Hosted by CFSI, the annual dinner benefits the mission of the non-profit policy organization, which is designed to educate members of Congress about fire and life safety issues. This year’s honored speakers were four co-chairs of the Congressional Fire Services Caucus: Senator Susan Collins (ME), Senator Tom Carper (DE), Congressman Steny Hoyer (MD05) and Congressman Bill Pascrell (NJ09).
The Excellence in Fire Service-Based EMS Awards Program was established in 2010 to recognize volunteer, career, and combination fire departments for excellence in and enhancements to the delivery of emergency medical services. By showcasing their best practices, the awards program provides ideas for other fire departments to consider implementing as they seek to improve their fire service-based EMS systems.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 including 9 of the top 10 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (PI). In studies with SpHb, reductions in blood transfusion** were observed,8,9 and when used with PVi, a reduction in 30-day mortality was observed.10 In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
**Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
8. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
9. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
10. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Root® and Kite™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root and Kite, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
CFSI
Bill Webb, Executive Director
Phone: (202)-371-1277
Email: bwebb@cfsi.org
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces Full Market Release of Root® Patient Monitor and Connectivity Hub with Kite™ Supplemental Display System
Irvine, California – April 10, 2017 – Masimo (NASDAQ: MASI) announced today the full market release of Root® with Kite™, a supplemental display system. Kite expands visibility of patient data for clinicians by allowing data from Root to be viewed on bigger screens, in customized configurations, in operating rooms, cardiac theaters, emergency rooms, and other venues.
Kite connects to Root via a wired or wireless connection on the same IP network and displays monitoring data from the connected device on a smart TV. Data from devices connected to Root – including the Radical-7® Pulse CO-Oximeter®, the wearable, tetherless Radius-7® Pulse CO-Oximeter, and optional monitoring modules such as SedLine® Brain Function Monitoring, O3™ Regional Oximetry, and ISA™ capnography solutions – can also be simultaneously displayed on a supplemental screen using Kite. The Kite supplementary display system can be customized to enable clinicians to view the monitoring parameters, waveforms, and other data they require for that patient and type of care or operation.
-
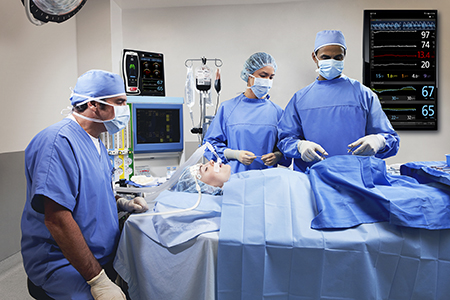
Masimo Root® Patient Monitor and Connectivity Hub with Kite™ Supplemental Display System
Masimo bedside monitoring devices provide clinicians with many types of data. During complex care, monitoring might involve so many different forms of real-time output, all of value to clinicians, that displaying them simultaneously on the primary device's smaller display may be inconvenient or impractical. With Kite, that data can be conveniently displayed on a large, easy-to-read monitor, relayed from the point-of-care device. For example, during endoscopy, while a patient is anesthetized, clinicians may wish to monitor with SedLine, for brain function, with rainbow Acoustic Monitoring® (RAM™), for respiration, and with ISA capnography, for end-tidal carbon dioxide, in addition to pulse oximetry and blood pressure. With Kite, the multiple data outputs from each of these types of monitoring can be simultaneously displayed on a large, centrally-located screen, in the clinicians' preferred configuration.
Dr. Don Marketto, D.O., Anesthesiologist, Mountain View Regional Hospital, Las Cruces, New Mexico, noted, "Masimo software made it extremely easy to adjust the placement and size of the information on the screen to the satisfaction of both the surgeon and anesthesiologist." Joseph Bremer, a perfusionist at Mountain View Regional Hospital, added, "Kite greatly enhanced the visibility of the Masimo monitor. During bypass I could easily view cerebral blood flow allowing time to validate adequate perfusion to my patient and maintain my attention on my bypass circuit."
"Kite showcases the versatility and flexibility of Root," said Joe Kiani, Founder and CEO of Masimo. "Our goal is to minimize clinician distraction and maximize focus on the patient and relevant data."
A promotional Kite opportunity is available for new and existing Masimo Root and Radical-7 customers.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring with Masimo Patient SafetyNet™* in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 including 9 of the top 10 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (PI). In studies with SpHb, reductions in blood transfusion** were observed,8,9 and when used with PVi, a reduction in 30-day mortality was observed.10 In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
**Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
8. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
9. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
10. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Root® and Kite™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root and Kite, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.



Massena Memorial Hospital of New York Adopts Masimo Root®
and Masimo Patient SafetyNet™
Hospital-Wide Supplemental Monitoring and Remote Clinician Notification System Automates Documentation of Patient Data
Massena, New York and Irvine, California – March 27, 2017 – Masimo (NASDAQ: MASI) announced today that Massena Memorial Hospital (MMH) of Massena, New York, has adopted Masimo Root® and Patient SafetyNet™*. The hospital is integrating Root with wearable rainbow SET™ Pulse CO-Oximeter® Radius-7® and Patient SafetyNet to implement a wireless monitoring, patient data automation, and supplemental remote clinician notification system that will be used in all medical-surgical units.
Masimo Patient SafetyNet is a supplemental remote monitoring and clinical notification system that enables information from bedside and tetherless wearable (when used with Radius-7) monitors, which use Masimo SET® and rainbow® noninvasive blood constituent monitoring technologies, to be accessible from different locations than patients, and relays alarm notifications to clinicians, wherever they may be. Masimo Measure-through Motion and Low Perfusion™ SET® pulse oximetry addresses the challenges of low perfusion and motion artifact that limit conventional pulse oximetry, and has been shown to significantly reduce false alarms and increase true alarm detection,1 helping allow clinicians to focus on the patients and alarms that need the most attention. In 2016, Dartmouth-Hitchcock Medical Center, which has been using Masimo SET® pulse oximetry and Patient SafetyNet as part of a comprehensive alarm management strategy in all medical-surgical units for ten years, reported achieving a 50% reduction in unplanned ICU transfers and a 60% reduction in rescue events over those ten years, despite increases in patient acuity and occupany.2
Another important feature to MMH is the ability of Root, in conjunction with Patient SafetyNet, to automate the transfer of patient vital signs, including temperature and blood pressure, to the hospital's Electronic Medical Record (EMR) system, which may help improve nursing workflows.
"The Masimo vital sign monitoring system will provide many benefits to the patients and staff of the Medical/Surgical/Pediatric unit," said Lisa Susice, MSN, RN, ICU/Med-Surg/Pediatric Nursing Director, MMH. "To be able to combine the ability to monitor our active patients in medical-surgical units reliably with the time-saving ability to transmit pulse rate, temperature, blood pressure, breathing rate, and oxygen saturation values automatically into the patients' electronic medical records allows our nurses to spend more time nursing our patients, instead of scribing and potentially being delayed in responding to patient alarms. Our staff, who have previously had to manually transcribe the information from the vital sign machine to a paper record, then eventually key it into the electronic record, will save many steps. Additionally, staff will be able to have a quick, real-time glance at each connected patient's pulse, respirations, and oxygen saturation from monitors strategically placed throughout the floor."
Ralene North, Chief Nursing Officer, MMH, added, "We are again excited to invest in technology that is focused on patient safety and will improve the working conditions of our nurses. The less time a nurse spends dealing with false alarms or entering data into the system, the more time that can be spent at the bedside attending to the personal and educational needs of the patient."
Root with Noninvasive Blood Pressure and Temperature is available with Radical-7® or Radius-7 Pulse CO-Oximeters. Radius-7 provides continuous tetherless wearable monitoring so that patients can have freedom of movement while being monitored. Monitoring parameters from Radical-7 or Radius-7 are sent to Patient SafetyNet through Root, allowing for hospital-wide remote monitoring, automated documentation of patient data in the EMR, and supplemental remote clinician notification of alerts and alarms.
"Massena Memorial Hospital has long been a valued Masimo customer, and with this additional investment, they are continuing to focus on patient safety," stated Joe Kiani, Founder and CEO of Masimo. "Continuous monitoring in the post-surgical ward has been shown to reduce preventable death.3 In addition, automating the transmission of vital signs data can help to improve workflow and reduce incomplete and erroneous medical records. We applaud Massena Memorial Hospital for leading the way in patient safety."
MMH is a full-service 50-bed acute-care community hospital located in northern New York with a medical staff of over 50 physicians in over 15 specialties. With more than 400 healthcare employees and 6 outreach clinics, MMH is the second largest employer in the town of Massena.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Shah N et al. Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers. J Clin Anesth. 2012 Aug;24(5):385-91.
2. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
3. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,6 including 9 of the top 10 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (PI). In studies with SpHb, reductions in blood transfusion* were observed,8,9 and when used with PVi, a reduction in 30-day mortality was observed.10 In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Estimate: Masimo data on file.
7. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
8. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
9. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
10. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Root® and Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root and Patient SafetyNet, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces CE Marking of Rad-97™Pulse CO-Oximeter® and Connectivity Hub with Noninvasive Blood Pressure
Neuchatel, Switzerland – March 20, 2017 – Masimo (NASDAQ: MASI) announced today the CE marking of noninvasive blood pressure (NIBP) measurements for the Rad-97™ Pulse CO-Oximeter® and connectivity hub. Rad-97 offers Measure-through Motion and Low Perfusion™ SET® pulse oximetry and upgradeable rainbow® noninvasive blood constituent monitoring technology in a compact standalone monitor configuration which can also collect and remotely transmit data from connected devices.
Rad-97 with NIBP enables clinicians to measure arterial blood pressure for adult, pediatric, and neonatal patients, with three measurement modes: spot-check, automatic interval (which measures blood pressure routinely, at a desired interval), and stat interval (which continually measures blood pressure for a desired duration). An integrated port allows clinicians to connect a blood pressure cuff inflation hose directly to Rad-97, and is compatible with both disposable and reusable cuffs, for a variety of patient types, designed for reliability and patient comfort.
-

Masimo Rad-97™ Pulse CO-Oximeter® and Connectivity Hub with Noninvasive Blood Pressure
With the addition of NIBP to Rad-97, clinicians can easily and automatically chart blood pressure data directly from the same monitoring device that measures oxygen saturation, total hemoglobin, and other noninvasive parameters. In hospital settings and when used in conjunction with Masimo Iris Gateway™ or Patient SafetyNet™*, data from Rad-97 and other devices connected via the Iris™ hub can be sent directly to the patient’s electronic medical record (EMR). With Patient SafetyNet, alarms and alerts from connected devices are seamlessly forwarded to clinicians.
Rad-97 includes built-in wireless connectivity, via Wi-Fi and Bluetooth®. Using Bluetooth or a wired USB connection, Rad-97 can connect to nearby devices, such as glucometers and weight scales, and can allow the data from the connected device to be transmitted remotely. Rad-97 will be available for use on home and enterprise networks to connect to remote monitoring systems, including Patient SafetyNet. Additional devices can be simultaneously attached to Rad-97 using the Iris hub.
Rad-97 features a high-resolution, 1080p HD color display with user-friendly multi-touch navigation, allowing clinicians to easily customize the device to best suit their monitoring and viewing needs. Users can also rapidly configure the device to accommodate different patient populations using customizable profiles. A rechargeable battery lasting seven hours allows Rad-97 to be used in situations where portability or extended operation without access to power are needed. An optional roll stand allows for tetherless device transport, offering additional flexibility when space is limited.
Rad-97 will also be available with an optional camera, which can be used in conjunction with Masimo Patient SafetyNet. The camera will provide a high resolution, high-frame rate video feed, as well as audio, to the Patient SafetyNet view-station. When used at home, camera-equipped Rad-97 will allow patients and clinicians to interact remotely, making it well-suited as a point-of-care device for potential telehealth applications.
Like Radical-7®, Rad-97 features Measure-through Motion and Low Perfusion pulse oximetry (SpO2), pulse rate (PR), and perfusion index (PI). Clinicians can add other monitoring solutions such as the rainbow SET™ measurements PVi®, total hemoglobin (SpHb®), methemoglobin (SpMet®), acoustic respiration rate (RRa®), carboxyhemoglobin (SpCO®), and oxygen content (SpOC™). Additional parameters such as Oxygen Reserve Index™ (ORi™) and respiration rate from the pleth (RRp™) are also available, making Rad-97 the smallest Masimo bedside device currently capable of monitoring the full rainbow SET platform.
"Rad-97 brings our core SET® and rainbow® technologies to a compact design, which allows broader applications in many new settings, including the home, with its hub and telepresence capabilities," said Joe Kiani, Founder and CEO of Masimo. "We're excited to extend its range of possibilities with the addition of integrated blood pressure measurement."
Rad-97 is not available in the United States.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (PI). In studies with SpHb, reductions in blood transfusion* were observed,6,7 and when used with PVi, a reduction in 30-day mortality was observed.8 In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
7. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
8. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Rad-97®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-97, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Evaluates the Utility of Masimo SpHb® Monitoring During Liver Transplantation
Irvine, California – March 13, 2017 – Masimo (NASDAQ: MASI) announced today the publication of a recent study conducted on adult patients undergoing liver transplantation (LT), in which researchers assessed the accuracy and trending ability of noninvasive Masimo SpHb® (total hemoglobin) measurement, as compared to conventional laboratory hemoglobin (HbL) measurements. The researchers found that SpHb had "clinically acceptable accuracy of hemoglobin measurement [compared] with a standard laboratory device when used during LT" and that "[t]his technology can be useful as a trend monitor during all surgical phases of LT and can supplement HbL to optimize transfusion decisions or to detect occult bleeding."1
Monitoring a patient's hemoglobin (Hb) levels is essential during LT because, as the researchers point out, "a serious loss of blood and fluid shift causes great variations [in] the Hb level, and delayed blood transfusion may cause graft dysfunction because of hypo-perfusion and tissue hypoxia. Moreover, more importantly, over-transfusion is associated with end organ damage and graft dysfunction." Recognizing that the "benefits and clinical advantages of noninvasive, rapid, and accurate determination of Hb in the operating room are obvious," Dr. Kayhan and colleagues sought to evaluate the accuracy and trending ability of Masimo SpHb during LT by comparing its measurements to those of a standard laboratory hematology analyzer.
To this end, the investigators enrolled fifty-five adult patients undergoing orthotopic LT in the study. The patients' Hb levels were analyzed using a Beckman Coulter LH 780 Hematology Analyzer, with each patient's arterial blood being sampled six times, twice during each of the three phases of the surgery: pre-anhepatic, anhepatic, and neohepatic. SpHb values were recorded within 10 seconds of each blood sample using a Masimo Radical-7® Pulse CO-Oximeter® (software version 7.8.0.1) and Masimo rainbow® ReSposable R2-25r and R2-25a sensors. A total of 282 paired measurements were collected and analyzed. HbL values ranged from 5.4 to 17.1 g/dL (mean 10.58) and SpHb values ranged from 6.9 to 17.7 g/dL (mean 11.44).
To compare the accuracies of the two methods, the researchers used a Bland-Altman plot and calculated absolute bias (the differences between SpHb and HbL) of 0.86 (95% CI = 0.50-1.21), precision (one standard deviation of the bias) of 1.58, and limits of agreement of -2.25 to 3.96. Using Pearson's correlation analysis, the researchers found that the correlation between the two sets of values was "highly significant": Pearson's correlation coefficient r=0.73; 95% confidence interval = 0.67-0.78, R2=0.53, p less than 0.001.
The researchers concluded that "[t]he results of this study show that SpHb has clinically acceptable accuracy of Hb measurement as compared with a standard laboratory device when used during LT. This technology may provide more timely information on Hb status than intermittent blood sample analysis and thus has the potential to improve blood management during LT. The trending accuracy may not only detect occult bleeding but can also prevent over-transfusion after bleeding; at least this method has the potential to supplement detection of changes. Nevertheless, due to underestimation in the lower Hb values, clinicians should be cautious when making decisions based on SpHb alone. Instead of focusing on a single value, SpHb may be considered an early warning system and a trend monitor. Future studies should evaluate the utility of SpHb in terms of overall clinical outcomes of transfusion decision."
"In prior studies using SpHb monitoring, reductions in blood transfusion were observed,2,3,4 and when used with PVi®, another Masimo noninvasive measurement, a reduction in 30-day mortality was observed5," stated Joe Kiani, Founder and CEO of Masimo. "Dr. Kayhan's study adds to the evidence that SpHb may be a useful tool during procedures such as liver transplantation."
SpHb monitoring may provide additional insight to the directional trend of hemoglobin between invasive blood samples. SpHb monitoring is intended to supplement, not replace, laboratory measurements. Blood samples should be analyzed by laboratory instruments when possible prior to clinical decision making.
References
1. Kayhan et al. Accuracy of Noninvasive Hemoglobin Monitoring by Pulse CO-Oximeter During Liver Transplantation. Minerva Anestesiologica. 2017 Jan 20. DOI: 10.23736/S0375-9393.17.11652-4
2. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedia Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
3. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
4. Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong, 2016. Abstract #PR607.
5. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (PI). In studies with SpHb, reductions in blood transfusion* were observed,6,7 and when used with PVi, a reduction in 30-day mortality was observed.8 In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
7. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
8. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces Availability of the RD SedLine® Adult EEG Sensor
Irvine, California – March 6, 2017 – Masimo (NASDAQ: MASI) announced today availability of the RD SedLine® EEG sensor, for use with Masimo SedLine Brain Function Monitoring and compatible with simultaneous use of Masimo O3™ Regional Oximetry. SedLine and O3 provide simultaneous monitoring on the Masimo Root® monitoring platform, helping to give clinicians more information about the brain.
-
Masimo RD SedLine® Adult EEG Sensor
The RD SedLine EEG sensor features a repositioned, color-coded sensor-cable connection that lies comfortably on the patient's head and soft foam pads to reduce discomfort upon application to the patient. The sensor's streamlined shape and built-in fitting guide allow simultaneous application of SedLine and O3 sensors. The sensor’s performance and specifications remain the same and work with existing SedLine modules, via an updated patient cable.
SedLine brain function monitoring features four simultaneous EEG leads to enable continuous assessment of both sides of the brain, four EEG waveforms, a Density Spectral Array (DSA; an easy-to-interpret, high-resolution display of bi-hemispheric activity and EEG power), and the Patient State Index (PSI; a processed EEG parameter related to the effect of anesthetic agents). Next Generation SedLine, available outside the U.S., enhances the PSI to make it less susceptible to electromyographic (EMG) interference and to improve performance in low-power EEG cases.
-
Masimo RD SedLine® Adult EEG and O3™ Regional Oximetry Sensors
O3 regional oximetry uses near-infrared spectroscopy (NIRS) to continuously monitor absolute and trended regional tissue oxygen saturation (rSO2) in the cerebral region. Regional oximetry may help clinicians monitor cerebral oxygenation in situations in which pulse oximetry alone may not be fully indicative of the oxygen in the brain due to various factors, such as the type of clinical procedure being performed.
Dr. David Drover, Professor of Anesthesiology in the Department of Anesthesiology, Perioperative and Pain Medicine at Stanford Hospital, stated, "The RD SedLine sensor allows simultaneous application with O3 Regional Oximetry to deliver more information about my patient's brain in a single specialty monitor."
"Root with SedLine and O3 presents a powerful brain monitoring solution," said Joe Kiani, Founder and CEO of Masimo. "With the addition of the RD SedLine EEG sensor, the EEG and optical sensors fit together like puzzle pieces, making it easier for clinicians to simultaneously monitor patients with both technologies, while providing a comfortable experience for the patient."
The RD SedLine EEG sensor is available in the U.S. Next Generation SedLine does not have 510(k) clearance and is not available in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (PI). Studies with SpHb have shown reductions in unnecessary blood transfusion*,6,7 and when used with PVi, reductions in length of hospital stay8 and 30- and 90-day mortality.9 In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
7. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
8. Thiele RH et al. Standardization of Care: Impact of an Enhanced Recovery Protocol on Length of Stay, Complications, and Direct Costs after Colorectal Surgery. JACS. 2015. doi: 10.1016/j.jamcollsurg.2014.12.042.
9. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine®, RD SedLine EEG sensors, and O3™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine, RD SedLine EEG sensors, and O3, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Assesses the Utility of Masimo PVi® Monitoring During Colorectal Surgery
Neuchatel, Switzerland – February 27, 2017 – Masimo (NASDAQ: MASI) announced today the findings of a recent study conducted on low-risk patients undergoing colorectal surgery, in which researchers assessed the utility of noninvasive and continuous Masimo PVi® (Pleth Variability Index) monitoring to guide fluid management, as compared to esophageal Doppler, an invasive method. The researchers found no significant difference between the two technologies in mean total fluid administered and concluded that "PVi offers an entirely noninvasive alternative for goal-directed therapy in this group of patients."1
In the study, Dr. Warnakulasuriya and colleagues at York Teaching Hospital in York, United Kingdom, evaluated the performance of Masimo PVi monitoring in guiding fluid management, as compared to that of an established technology, esophageal Doppler. Forty low-risk patients undergoing elective colorectal surgery were enrolled in the study. The patients were randomly assigned to two groups, with each group having fluid therapy directed by one of the two technologies. The researchers measured the absolute volume of fluid given intraoperatively and fluid volume at 24 hours. The researchers found "no significant difference between PVi and esophageal Doppler groups in mean total fluid administered (1286 vs 1520 ml, p=.300) or mean intraoperative fluid balance (+839 v + 1145 mL, p=.150)."
The researchers concluded that "amongst fit patients undergoing major colorectal surgery there was no significant difference in the volume of fluid administered when targeted by noninvasive PVi technology compared to a stroke volume maximization technique using esophageal Doppler. There was no significant difference in postoperative outcomes between the groups. Therefore, PVi offers a noninvasive, consumable free alternative for intraoperative fluid optimization in fit patients undergoing major colorectal surgery, where intraoperative goal-directed therapy is deemed a standard of care but there is no requirement for arterial cannulation."
PVi is a measure of the dynamic changes in perfusion index (PI) that occur during the respiratory cycle. In other clinical studies, PVi has been shown to provide benefits in the monitoring of mechanically-ventilated patients under general anesthesia during surgery,2,3,4,5 in the ICU in both adults and children,6,7 and in septic patients in the early stages of shock in the emergency department.8 Another study used PVi as part of goal-directed therapy for patients in an enhanced recovery after surgery (ERAS) program who underwent colorectal surgery; the program led to significant reductions in lengths of stay, costs, surgical site infections, fluid administered, as well as improvement in patient satisfaction.9 In a study in which PVi was used in conjunction with Masimo SpHb® (noninvasive hemoglobin measurement), the technologies were shown to reduce mortality at 30 and 90 days.10
"Clinical evidence for the utility of Masimo PVi continues to amass," said Joe Kiani, Founder and CEO of Masimo. "Dr. Warnakulasuriya’s study provides additional information about the benefits of PVi. We are grateful for the opportunity we have to continue to improve patient outcomes and reduce cost of care with our innovative noninvasive monitoring."
References
1. Warnakulasuriya S et al. Comparison of esophageal Doppler and plethysmographic variability index to guide intraoperative fluid therapy for low-risk patients undergoing colorectal surgery. Journal of Clinical Anesthesia. (2016)34,600-608.
2. Cannesson M et al. Pleth Variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre. Br J Anaesth. 2008;101(2):200-6.
3. Zimmermann M et al. Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically Ventilated Patients Undergoing Major Surgery. Eur J Anaesthesiol. 2010 Jun;27(6):555-61.
4. Fu Q et al. Stoke Volume Variation and Pleth Variability Index to Predict Fluid Responsiveness During Resection of Primary Retroperitoneal Tumors in Han Chinese. Biosci Trends. 2012 Feb;6(1):38-43.
5. Haas S et al. Prediction of Volume Responsiveness using Pleth Variability Index in Patients Undergoing Cardiac Surgery after Cardiopulmonary Bypass. J Anesth. 2012 Oct;26(5):696-701.
6. Loupec T et al. Pleth Variability Index Predicts Fluid Responsiveness in Critically Ill Patients. Crit Care Med. 2011;39(2):294-299.
7. Byon HJ et al. Prediction of Fluid Responsiveness in Mechanically Ventilated Children Undergoing Neurosurgery. Br J Anaesth. 2013 Apr;110(4):586-91.
8. Feissel M et al. Plethysmographic Variation Index Predicts Fluid Responsiveness in Ventilated Patients in the Early Phase of Septic Shock in the Emergency Department: A Pilot Study. J Crit Care. 2013 May 14:634-639.
9. Thiele et al. Standardization of Care: Impact of an Enhanced Recovery Protocol on Length of Stay, Complications, and Direct Costs after Colorectal Surgery. Journal of the American College of Surgeons (2015). doi: 10.1016/j.jamcollsurg.2014.12.042.
10. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to helps clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (PI). Studies with SpHb have shown reductions in unnecessary blood transfusion*,6,7 and when used with PVi, reductions in length of hospital stay8 and 30- and 90-day mortality.9 In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
7. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
8. Thiele RH et al. Standardization of Care: Impact of an Enhanced Recovery Protocol on Length of Stay, Complications, and Direct Costs after Colorectal Surgery. JACS. 2015. doi: 10.1016/j.jamcollsurg.2014.12.042.
9. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo PVi®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo PVi, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces Recent Study Monitoring Methemoglobin Levels During Administration of Inhaled Nitric Oxide
Irvine, California – February 21, 2017 – Masimo (NASDAQ: MASI) announced today the findings of a recent study conducted on children admitted to a Ugandan hospital with fever and malaria, in which Masimo's noninvasive measurement SpMet® was used to monitor methemoglobin (MetHb) levels. One group of children with severe malaria was selected to receive inhaled nitric oxide (iNO) treatment as an adjunct to intravenous therapy while a placebo group received room air. Both of these groups were monitored with SpMet.1
Red blood cells containing hemoglobin can become oxidized in the presence of certain drugs and compounds, including nitric oxide, changing it to methemoglobin (MetHb), which impairs the oxygen-carrying capacity of blood. When MetHb levels rise, headache, respiratory distress, cyanosis, and finally death may occur. A 2004 study conducted at Johns Hopkins Hospital reported that 20% of the patients tested, from neonates to geriatrics, had elevated MetHB caused by the side effects of 40 drugs given to patients in hospitals, including nitric oxide. Three patients nearly died and one patient died from elevated MetHb during the study period.2
An estimated 1.2 million people die from malaria annually worldwide, with a mortality rate of 8-20% in children with severe malaria.3 In the Ugandan study, Dr. Andrea Conroy and colleagues at Jinja Regional Referral Hospital assessed an adjunctive therapy for malarial patients: the administration of inhaled nitric oxide (iNO) – but as iNO is absorbed by the body, it "induces MetHb in a dose-dependent manner." Noting that "[t]here are no reliable estimates of methemoglobinemia in low resource clinical settings," but seeking to "evaluate whether iNO could improve clinical recovery...in a cohort of children with severe malaria," the investigators chose to monitor MetHb levels during treatment with a Masimo Rad-57® Pulse CO-Oximeter® with noninvasive SpMet monitoring.
The investigators in Uganda selected 180 children admitted to the hospital with severe malaria between 2011 and 2013 to receive either iNO (n=88) or a placebo, room air (n=92), in conjunction with standard anti-malarial treatment. MetHb levels were measured on a four-hourly basis following gas initiation, using Masimo SpMet. Between gas initiation and the first check with SpMet, MetHb levels rose from an average of 1.8% to 4.1% for the iNO group but stayed the same (1.7% to 1.8%) in the placebo group. MetHb levels typically plateaued within 12-24 hours of receiving iNO. Gas was withdrawn for 31 children (placebo: 12; iNO: 19; p=0.13).
The researchers stated that "we were able to evaluate the variability in MetHb responses within subjects and the frequency of methemoglobinemia prompting study gas discontinuation. Despite the high doses of iNO administered, study gas was temporarily discontinued only five times for MetHb greater than 10% (all children in the iNO group). We were able to re-start study gas for all children that had a MHb measurement that exceeded 10% once the MetHb returned to less than 7% without having the MetHb exceed 10% again. It was not necessary to wean children off iNO, in contrast to studies administering iNO to neonates with hypoxic respiratory failure, as we did not observe any rebound effects (e.g. worsening oxygenation) following discontinuation of study gas."
The authors concluded that, "Hospitalized children with evidence of impaired oxygen delivery, metabolic acidosis, anemia, or malaria were at risk of methemoglobinemia. However, we demonstrated high-dose iNO could be safely administered to critically ill children with severe malaria with appropriate MHb monitoring."
Joe Kiani, Founder and CEO of Masimo, stated, "It's great to see that our invention of continuous methemoglobin monitoring has allowed these clinicians to study the outcomes of administering iNO treatment. We hope to continue developing monitoring technologies that help to address such public health crises."
SpMet monitoring is not intended to be used as the sole basis for making diagnosis or treatment decisions. It is intended to be used in conjunction with other clinical tools, including signs and symptoms and laboratory blood tests.
References
1. Conroy et al. Methemoglobin and nitric oxide therapy in Ugandan children hospitalized for febrile illness: results from a prospective cohort study and randomized double-blind placebo-controlled trial. BMC Pediatrics. (2016) 16:177. DOI 10.1186/s12887-016-0719-2.
2. Ash-Bernal et al. Acquired methemoglobinemia: A retrospective series of 138 cases at 2 teaching hospitals. Medicine. October 2004;83(5)265-73. DOI 10.1097/01.md.000141096.00377.3f.
3. Murray et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379(9814):413-31.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to helps clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (PI). Studies with SpHb have shown reductions in unnecessary blood transfusion*,6,7 and when used with PVi, reductions in length of hospital stay8 and 30- and 90-day mortality.9 In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
7. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
8. Thiele RH et al. Standardization of Care: Impact of an Enhanced Recovery Protocol on Length of Stay, Complications, and Direct Costs after Colorectal Surgery. JACS. 2015. doi: 10.1016/j.jamcollsurg.2014.12.042.
9. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpMet®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpMet, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces CE Marking of Respiration Rate Measurement on MightySat™ Rx
Masimo MightySat Rx is the First Fingertip Pulse Oximeter to Measure Respiration Rate
Neuchatel, Switzerland – February 15, 2017 – Masimo (NASDAQ: MASI) announced today the the CE marking of the measurement of respiration rate from the pleth (RRp™) on the MightySat™ Rx fingertip pulse oximeter. MightySat Rx is a noninvasive device that measures and displays functional oxygen saturation (SpO2), Pulse Rate (PR) and Perfusion Index (PI) with the option to add Pleth Variability Index (PVi®) and now, RRp.
-
Masimo MightySat™ with RRp™
Respiration rate, or the number of breaths taken per minute, typically requires manually counting breaths with a timer and then converting to a per minute rate, or being fitted with chest leads or straps that can be inconvenient. With the addition of RRp to MightySat Rx, respiration rate can conveniently be measured using the same fingertip sensor that measures SpO2, PR, PI, and PVi (a measurement of the dynamic changes in PI that occur during the respiratory cycle). RRp is measured only when the respiratory movement-induced signal is present in the pulsatile waveform and may not be available during certain conditions, such as very irregular breathing and excessive movement.
MightySat Rx is indicated for use with both adult and pediatric patients during both no motion and motion conditions, who are well or poorly perfused, in hospitals, hospital-type facilities, mobile, and home environments. It offers a Bluetooth wireless interface to the Masimo Professional Health mobile application to track, trend, and communicate measurements. MightySat Rx features the same Measure-through Motion and Low Perfusion™ SET® pulse oximetry available in a variety of bedside Masimo and OEM monitors. Masimo SET® addresses the challenges of low perfusion and motion artifact that limit conventional pulse oximetry by harnessing the power of adaptive filters to reduce measurement inaccuracy. Infection control issues aside, Masimo SET® performance benefits are maximized by choosing the the correct sensor type for the applicable use scenario: adhesive sensors for continuous monitoring, reusable cabled sensors for short-term monitoring and MightySat Rx fingertip oximeters for spot-checks on those who are not moving excessively and do not have very poor perfusion. Masimo SET® helps clinicians monitor oxygen saturation and pulse rate during motion and low perfusion for more than 100 million patients a year1 and is the primary pulse oximetry at top hospitals, including 9 of the top 10 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.2
"MightySat Rx is our smallest, most compact pulse oximeter, and as such is particularly versatile, offering the convenience of portability," stated Joe Kiani, Chairman and CEO of Masimo. "We are happy to be able to increase its capability with the addition of RRp, and to continue innovating in the field of mobile monitoring devices for the professional caregiver market."
RRp does not have 510(k) clearance and is not available in the U.S.
References
1. Estimate: Masimo data on file.
2. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to helps clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (PI). Studies with SpHb have shown reductions in unnecessary blood transfusion*,6,7 and when used with PVi, reductions in length of hospital stay8 and 30- and 90-day mortality.9 In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
7. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
8. Thiele RH et al. Standardization of Care: Impact of an Enhanced Recovery Protocol on Length of Stay, Complications, and Direct Costs after Colorectal Surgery. JACS. 2015. doi: 10.1016/j.jamcollsurg.2014.12.042.
9. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo MightySat™ Rx and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo MightySat Rx and SET®, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces the Addition of Early Warning Score to the Root® Patient Monitoring and Connectivity Platform
Neuchatel, Switzerland – February 1, 2017 – Masimo (NASDAQ: MASI) announced today the limited market release of Early Warning Score (EWS) on the Root® patient monitoring and connectivity platform. EWS aggregates information from multiple vital signs and clinical observations to generate a score that represents the potential degree of patient deterioration.
Root, which works in conjunction with Radical-7® or Radius-7® Pulse CO-Oximeters® and Masimo Open Connect™ (MOC-9™) measurements, features Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, rainbow SET™ pulse CO-Oximetry, Nomoline™ capnography and gas monitoring, SedLine® brain function monitoring, O3™ regional oximetry, and SunTech® blood pressure and Welch Allyn® temperature monitoring. Masimo SET® helps clinicians monitor oxygen saturation and pulse rate during motion and low perfusion for more than 100 million patients a year1 and is the primary pulse oximetry technology at top hospitals, including 9 of the top 10 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.2
Patient data from Radical-7 or Radius-7 and data collected using Root and other connected Masimo and third-party devices can be shared with Masimo Patient SafetyNet™*, providing hospital-wide remote monitoring and clinician notification, as well as the ability to automatically push patient data to a hospital’s Electronic Medical Record (EMR). Each time a clinician pushes data to the EMR via Root connected to Patient SafetyNet, an Early Warning Score (EWS) can now be included. Clinicians can also choose to have the standalone Root perform EWS calculations.
There are several EWS protocols, such as Pediatric Early Warning Score (PEWS), Modified Early Warning Score (MEWS), and National Early Warning Score (NEWS). These various scores require vital signs contributors – such as oxygen saturation, pulse rate, respiration rate, body temperature, and systolic blood pressure – and contributors input by clinicians, such as level of consciousness, use of supplemental oxygen, and urine output. The weighting and number of contributors differ depending upon which EWS protocol is used. Root can be customized for various predefined EWS protocols, or hospitals can configure their own set of required contributors, and their relative weights, to create an EWS unique to their care environment.
Recent peer-reviewed studies, across care areas, have suggested that the use of NEWS may have clinical benefits: Vanamali et al. notes that NEWS is a "useful simple physiological scoring system for assessment and risk management of medical emergency admissions."3 Smith et al. found that an EWS of 5 or greater after laparotomy is associated with adverse outcomes, while recommending that future studies evaluate the ability of EWS to predict and prevent such outcomes.4
Outside the U.S., as part of the Patient SafetyNet platform, Masimo also offers Halo Index™. Whereas EWS provides a spot-check score using the NEWS standard, Halo Index presents a dynamic, cumulative trending assessment of global patient status as a single displayed number ranging from 0 to 100. Halo Index uses available Masimo parameters from connected monitoring devices, but is scalable to include additional information from the patient data repository. Masimo designed Halo Index to mimic the systematic approach that expert clinicians use in assessing patient physiologic deterioration, analyzing the patient’s history and extracting key vital sign parameter characteristics; increases in a patient’s Halo Index may indicate the need for clinicians to more closely assess the patient.
"Root, from its versatile connectivity options to its advanced patient monitoring, from rainbow® SpHb® to SET® SpO2, has long been helping hospitals improve and automate their patient care. Now, with Early Warning Score, Root can help clinicians stay ahead of the care race and transfer their patients home safely," said Joe Kiani, Founder and CEO of Masimo.
Root with Early Warning Score (EWS) and Halo Index are not available in the U.S. EWS is a convenient aid to clinical assessment and not a substitute for clinical judgement.
*The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Estimate: Masimo data on file.
2. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
3. Vanamali DR, et al. The Role of National Early Warning Score (News) in Medical Emergency-Patients in Indian Scenario: A Prospective Observational Study. Journal of Evolution of Medical and Dental Sciences. 2014; Vol. 3, Issue 13, March 31; Page: 3524-3528, DOI: 10.14260/jemds/2014/2315.
4. Smith, et al. Early warning score: An indicator of adverse outcomes in postoperative patients on a gynecologic oncology service. Gynecol Oncol. 2016 Oct;143(1):105-8. doi: 10.1016/j.ygyno.2016.08.153. Epub 2016 Aug 6.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® has also been shown to helps clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring in post-surgical wards, reduce rapid response activations and costs.3,4,5 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (PI). Studies with SpHb have shown reductions in unnecessary blood transfusion*,6,7 and when used with PVi, reductions in length of hospital stay8 and 30- and 90-day mortality.9 In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface, enabling other companies to augment Root with new features and measurement capabilities. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor, iSpO2® pulse oximeter for smartphones, and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
*Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
7. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
8. Thiele RH et al. Standardization of Care: Impact of an Enhanced Recovery Protocol on Length of Stay, Complications, and Direct Costs after Colorectal Surgery. JACS. 2015. doi: 10.1016/j.jamcollsurg.2014.12.042.
9. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root, contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Reaffirms Commitment to India with Launch of Advanced Monitoring Technologies Made for India
Leading Bangalore Hospital CEOs Convene for Masimo Roundtable
Bangalore, India – January 2, 2017 – Masimo (NASDAQ: MASI) announced today the launch and availability of the Rad-97™ Pulse-CO Oximeter®* and Next Generation Sedline®* Brain Function Monitoring in India. The announcement was made in Bangalore by Joe Kiani, Founder and CEO of Masimo, at a roundtable of the CEOs of top Bangalore hospitals.
Joe Kiani, Founder and CEO, Masimo, commented, "The Indian healthcare sector recognizes the need for technologies that help their clinicians get the best results, the first time. Masimo's noninvasive patient monitoring technology innovations offer capabilities that have never been possible before. With a large population and a burgeoning demand for an improved quality of life – including the safe, high-quality healthcare to which all are entitled – India continues to be a focus market for Masimo. We will continue to invest in India and strive to make our technologies and products as accessible as possible, as evidenced by the new Rad-97 and Next Generation SedLine."
Bharat Monteiro, Masimo Country Manager for India, noted, "Masimo is committed to doing what is best for patient care in India. In the course of our engagement with leading Indian hospitals, we have witnessed an increased awareness of and commitment to patient safety requirements. Medical providers across the country are eager to adopt advanced technologies and monitoring devices, such as Rad-97 and Next Generation SedLine, which we hope will help provide access to better healthcare, at lower cost."
Dr. Ashutosh Raghuvanshi, Managing Director and Group CEO of Narayana Health Hospitals, one of the participants in the roundtable, commented, "We constantly endeavor to improve the facilities at our hospitals to ensure better patient care and treatment outcomes. With the newly launched Rad-97, we will be able to use Masimo SET® pulse oximetry in more care areas, including ward monitoring, which will help facilitate better monitoring and patient treatment. Narayana Health performs the highest number of cardiac surgeries in India, and I believe that noninvasive hemoglobin (SpHb) will add immense value to complex cardiac surgeries, helping us reduce unwarranted transfusions and the risk of infection."
Rad-97 features Measure-through Motion and Low Perfusion™ SET® pulse oximetry, which studies have shown helps clinicians reduce severe retinopathy of prematurity in neonates,1 improve CCHD screening in newborns,2 and, when used for continuous monitoring in post-surgical wards, reduce rapid response activations and costs.3,4,5 Rad-97 also offers the same upgradeable rainbow SET™ technology as the Radical-7® Pulse CO-Oximeter, in a versatile, standalone monitor configuration. Using Rad-97, clinicians can monitor such rainbow® measurements as total hemoglobin (SpHb®) and PVi®. Studies with SpHb have shown reductions in unnecessary blood transfusion†,6,7 and when used with PVi, reductions in length of hospital stay8 and 30- and 90-day mortality.9 rainbow® can also measure methemoglobin (SpMet®), acoustic respiration rate (RRa®), carboxyhemoglobin (SpCO®), Oxygen Reserve Index™* (ORi™), and oxygen content (SpOC™). Rad-97 also features an integrated camera* for clinician tele-presence via Patient SafetyNet™‡ and a high-resolution 1080p HD color display with user-friendly multi-touch navigation, similar to Root® and Radical-7, allowing clinicians to easily customize the device to best suit their monitoring needs.
SedLine features four simultaneous EEG leads to enable continuous assessment of both sides of the brain, as well as a Density Spectral Array (DSA), an easy-to-interpret, high-resolution display of bi-hemispheric activity. Next Generation SedLine enhances Masimo’s processed EEG parameter, the Patient State Index (PSI), to make it less susceptible to electromyographic (EMG) interference and to improve performance in low-power EEG cases.
*Rad-97, the camera feature, Next Generation SedLine, and ORi do not have 510(k) clearance and are not available in the U.S.
†Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
‡The use of the trademark SafetyNet is under license from University HealthSystem Consortium.
References
1. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
2. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
3. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
4. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
5. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
6. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedia Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
7. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
8. Thiele RH et al. Standardization of Care: Impact of an Enhanced Recovery Protocol on Length of Stay, Complications, and Direct Costs after Colorectal Surgery. JACS (2015). doi: 10.1016/j.jamcollsurg.2014.12.042.
9. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/home/clinical-evidence/clinical-evidence.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Rad-97™ Pulse CO-Oximeter® and Next Generation SedLine® Brain Function Monitoring. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-97 and Next Generation SedLine, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Ajith Pai / Amrutha Moorthy
Adfactors PR (Masimo in India)
Phone: +91 96633 94732
Email: ajit.pai@adfactorspr.com / amrutha.moorthy@adfactorspr.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

