News & Media-2016
Home / 2016
2016
NEWS & MEDIA:

Masimo Announces FDA 510(k) Clearance for TFA-1™ Single-Patient-Use Forehead Sensor
Irvine, California – December 19, 2016 – Masimo (NASDAQ: MASI) announced today FDA 510(k) clearance for the TFA-1™ Single-Patient-Use Adhesive Forehead Sensor. This single-patient-use sensor allows clinicians to monitor patients using Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry from an alternative monitoring site, the forehead, rather than a finger.
-
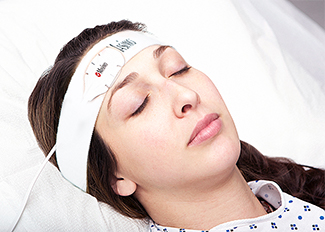
Masimo TFA-1™ Single-Patient-Use Forehead Sensor
Forehead sensors may provide oxygen saturation measurements that are less susceptible to peripheral perfusion changes, and were shown in a 2004 study to have faster detection of desaturation and resaturation, as compared to digit sensors.1 Forehead sensors can also be easily accessed during surgery and resuscitation and on patients with finger deformities or whose fingers are not accessible. As a single-patient-use sensor, TFA-1 avoids the management complexities (cleaning, storage, and inter-department transport) that accompany reusable sensors.
Masimo SET® includes measurement of oxygen saturation (SpO2), pulse rate (PR), perfusion index (Pi), and PVi®, a measure of the dynamic changes in PI that occur during the respiratory cycle. Masimo SET® addresses the challenges of low perfusion and motion artifact that limit conventional pulse oximetry by harnessing the power of adaptive filters to reduce measurement inaccuracy. Masimo SET® helps clinicians monitor more than 100 million patients a year2 and is used by 8 of the top 10 hospitals listed in the 2016-17 U.S. News and World Report Best Hospitals Honor Roll.3
References
1. Redford DT et al. Intraoperative Perfusion Changes at Two Pulse Oximetry Monitoring Sites: The Digit vs. the Nare. Anesth Analg. 2004;98(2S)S-94.
2. Estimate: Masimo data on file.
3. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo TFA-1™ Sensors and SET® pulse oximetry. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo TFA-1 Sensors and SET® pulse oximetry, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Giving Back with Masimo
Irvine, California – December 6, 2016 – Masimo (NASDAQ: MASI) gives thanks, as always, for the many joys and successes that 2016 has brought us. It has nonetheless been a difficult year, both at home and abroad: millions of refugees, displaced from war-torn homelands, continue to suffer. In low-resource settings across the world, many of the most at-risk patients lack access to even the most basic medical care, such as oxygen. Brexit and a particularly contentious presidential election have left many people feeling more divided than united. And hospital patients around the world, even those lucky enough to have good medical care, continue to experience unnecessary harm and to die as a result of inadvertent mistakes and lapses in patient safety.
As in years past, in the spirit of giving back and of trying to make the world a better place for everyone, we humbly ask you, our loyal friends and customers, to help us to donate to some of what we believe to be the worthiest charities, representing a variety of causes. We simply ask that you send an email to charity@masimo.com, specifying your choice from the list below, and we will donate $10 in your name to that charity. We also encourage you to donate to them directly.
- AidRefugees – https://www.whitehouse.gov/campaign/aidrefugees
- Médecins Sans Frontières (MSF) International (Doctors without Borders) – http://www.doctorswithoutborders.org/our-work/humanitarian-issues/refugees-and-idps
- RED CROSS – https://www.icrc.org/en/where-we-work/middle-east/syria
- SAMS (Syrian American Medical Society) – https://www.sams-usa.net/foundation/
- UN Refugee Agency – http://www.unhcr.org/emergency/5051e8cd6-560953162.html
- UNICEF – http://www.unicef.org/emergencies/syria/
- Syrian Community Network – http://www.syriancommunitynetwork.org/
- SOS Children’s Villages – http://www.sos-childrensvillages.org/
- Smile Train – https://www.smiletrain.org/
- Patient Safety Movement Foundation – http://www.patientsafetymovement.org/
- Feeding America – http://www.feedingamerica.org/
As part of Masimo's commitment to giving back, in 2016 we have continued to focus on providing relief to refugees through donations to Doctors Without Borders, the Syrian Community Network, and the Syrian American Medical Society. Employee donations, matched by the Kiani family, have to date raised more than $200,000. Last year, we announced a $5 million medical equipment donation to Jordan, to help provide healthcare to over 1 million Syrian refugees, and work on that project has continued throughout 2016. In addition, this year we've formed several partnerships dedicated to advancing the quality of healthcare in low-resource settings around the world: with Smile Train, to promote safe cleft-lip and palate surgery; with a variety of non-profit organizations to create United for Oxygen, an alliance dedicated to increasing access to medical oxygen and pulse oximetry; and the Gates Foundation, to develop a low-cost pulse oximeter and promote pneumonia screening.
Joe Kiani, Founder and CEO of Masimo, notes: "Tragic headlines, bespeaking much loss and woe, have dominated the news cycle for many years now. It's time for us all to take steps to transform those headlines, so that rather than the negative, we can focus on the great work so many are doing to care for those in need around the world: helping refugees find safe and dignified refuge amongst us, eliminating preventable hospital deaths, and providing access to safe surgery for the billions who still don't have it. Please join us in trying to channel the energy of despair into forces for good. And thank you for being a part of our Masimo family. We are grateful to you all and wish you all a wonderful holiday season. Here's to a happier and healthier New Year!"
@MasimoInnovates || #Masimo
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Masimo pulse oximetry, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Receives Gates Foundation Grant to Develop Combined Pneumonia-Screening Device for High-Burden Settings
Irvine, California – November 11, 2016 – Masimo (NASDAQ: MASI) announced today, in advance of World Pneumonia Day tomorrow, that it has received a grant of $4.95 million from the Bill and Melinda Gates Foundation (BMGF). The grant will support Masimo's efforts to develop a low-cost pulse oximeter for use in pneumonia screening in several low-resource areas outside the U.S. While still in development, the device will likely measure oxygen saturation and respiration rate – two key parameters that would provide additional information to clinicians when screening potential pneumonia cases in high-burden settings. The grant will also support studying the clinical use environment in such settings to ensure that the device is user friendly and fit for its purpose. The grant may also support the education materials to promote proper training and use of the device at all literacy levels.
Pneumonia remains the single largest treatable infectious cause of death in children worldwide, causing over 900,000 deaths each year among children under 5 years of age.1 In a study funded by the BMGF Diagnostics Modelling Consortium, the researchers concluded that in settings where supplemental oxygen is available, the addition of pulse oximetry to standard integrated management of child illness protocols could reduce pneumonia mortality rates.2 More recently, the World Health Organization (WHO) has sponsored an ongoing multi-country study to further assess how to enhance community case management of pneumonia treatment.3
Enhancing patient screening is critically important to reducing the global burden of pneumonia. Moreover, enhanced screening may empower healthcare providers by supporting informed decisions related to pneumonia diagnosis and treatment, with the appropriate administration of antibiotics and oxygen therapy when needed. Masimo and BMGF hope that Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry technology can help better screen children in even the most challenging conditions.
Rasa Izadnegahdar, Senior Program Officer on the Pneumonia Team at BMGF, commented, "We are excited to partner with Masimo for the purpose of improving screening for pneumonia to allow improved referral decisions by health workers in low-resource settings. Evaluating a child for fast breathing is the cornerstone of pneumonia assessment and combining this with a reliable and effective approach to assessing oxygen saturation can have significant impact in ensuring children with pneumonia receive appropriate care. Our landscape review of pneumonia diagnostic aid technologies has clearly demonstrated the poor performance of existing diagnostic aids, particularly respiratory rate timers. Diagnostic aids with improved sensitivity and specificity that are adapted and designed for use by health workers and caregivers can make a significant impact on pneumonia treatment. We look forward to working with Masimo to adapt their precise and accurate technology to respond to the needs of health workers, caregivers, and children in countries where pneumonia burden and deaths remain unacceptably high."
Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry is used to monitor millions of patients around the world. This project demonstrates the company's strong commitment to reducing the unnecessarily high global burden of pneumonia by bringing its proven patient screening and monitoring technology to the areas of the world that need it most.
"We are gratified to be able to partner with BMGF, which recently joined us in co-founding the United for Oxygen Alliance to increase access to oxygen and pulse oximetry in Ethiopia, in supporting our efforts to help fight pneumonia," said Joe Kiani, Founder and CEO of Masimo. "We hope to bring a much-needed screening tool to areas of the world blighted by countless needless deaths that are due to an illness considered low-risk in the developed world."
@MasimoInnovates || #Masimo
References
1. Pneumonia Fact Sheet, World Health Organization (WHO), September 2016. http://www.who.int/mediacentre/factsheefts/fs331/en/.
2. Floyd J et al. Evaluating the impact of pulse oximetry on childhood pneumonia mortality in resource-poor settings. Nature. 2015 Dec 3;528(7580):S53-9.
3. World Health Organization (WHO), 2016.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo pulse oximetry. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo pulse oximetry, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.



Philips and Masimo Sign Multi-Year Business Partnership Agreement in Patient Monitoring and Select Therapy Solutions
- Multi-year business partnership combines Masimo's expertise in non-invasive sensor and signal processing technologies and Philips' expertise in integrated patient monitoring and therapy solutions
- Business partnership involves technology integration and marketing and sales cooperation in North America and certain markets in Asia and Europe
- Agreement ends all pending lawsuits between the two companies and, in addition to marketing and integration commitments, includes a cash payment of USD 300 million by Philips to Masimo
Amsterdam, the Netherlands and Irvine, California – November 7, 2016 – Royal Philips (NYSE: PHG; AEX: PHIA) and Masimo Corporation (NASDAQ: MASI) today announced a wide-ranging, multi-year business partnership involving both companies' innovations in patient monitoring and therapy solutions. The partnership includes joint marketing and sales programs in North America and certain markets in Asia and Europe for Masimo's non-invasive sensor technologies, such as its rainbow® and SET® platforms, in conjunction with Philips' patient monitoring and select therapy solutions. In addition, Philips will in the future integrate Masimo SedLine® brain function monitoring, O3™ regional oximetry, and Nomoline™ capnography technologies in certain Philips IntelliVue® monitors.
Philips is a global leader in patient monitoring solutions with a comprehensive product portfolio ranging from multi-parameter bedside monitors to wearable patient monitors combined with mobile applications and clinical decision support tools. With a primary focus on prediction and prevention of patient deterioration, these integrated solutions aim to support clinical workflow and caregiver efficiencies, and enhance patient care. In 2015, an estimated 275 million patients were monitored using Philips' patient monitoring solutions.
Sensor and signal processing technologies are an essential component of patient monitoring solutions, and Masimo is a prolific innovator in this field. Examples of Masimo's non-invasive sensor and signal processing technology innovations include Masimo SET® pulse oximetry, Masimo rainbow® Pulse CO-Oximetry and Masimo total hemoglobin (SpHb®) monitoring technology.
"This business partnership agreement marks an important day for us and our customers as two leaders in patient monitoring collaborate to develop solutions designed to enhance clinical outcomes and patient safety," said Frans van Houten, CEO of Royal Philips. "I am very satisfied that we have reached an agreement that is beneficial for both companies and that we have ended our legal disputes. Going forward, Philips and Masimo will completely focus on jointly delivering meaningful innovations to our customers, such as the integration of Masimo rainbow® technology across our IntelliVue® patient monitoring product range."
"It's wonderful to think that Masimo and Philips will be working together for the benefit of patients and clinicians around the world," said Joe Kiani, Chairman and CEO of Masimo. "I am delighted that we were able to reach this important agreement which allows us to focus on the future to deliver the solutions that our customers have been looking for."
In conjunction with the appropriate Philips patient monitoring platform, Masimo's rainbow SET™ technology analyzes multiple wavelengths of light to accurately measure total hemoglobin (SpHb), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®) and Pleth Variability Index (PVi®) non-invasively and continuously. Continuous monitoring of SpHb on a Philips monitor at the point of care provides clinicians with real-time visibility to changes in hemoglobin in between invasive blood samplings.
Anticipated cash flow and income consequences for Philips
As part of the business partnership agreement, Philips and Masimo have agreed to end all pending lawsuits between the two companies, which includes that Philips is released from paying the USD 467 million (approximately EUR 428 million) jury verdict that was awarded to Masimo in October, 2014. Philips has agreed to make a USD 300 million cash payment (approximately EUR 275 million) to Masimo in the fourth quarter of 2016; and to invest in the relationship by making certain marketing and product integration commitments over the coming years. Entering into the business partnership agreement has minimal impact on income from operations (EBIT) in the fourth quarter of 2016.
Anticipated cash flow and income consequences for Masimo
As the result of anticipated legal fee savings during the fourth fiscal quarter, Masimo now expects its fiscal 2016 GAAP earnings per diluted share, exclusive of the impact of the business partnership agreement, to be $2.14, up from $2.13. Masimo expects to use some of the after-tax proceeds from the business partnership agreement to repay amounts outstanding under its revolving line of credit. The guidance set forth above is an estimate only and actual performance could differ.
@MasimoInnovates || #Masimo
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips' health technology portfolio generated 2015 sales of EUR 16.8 billion and employs approximately 70,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements - Royal Philips
This release may contain certain forward-looking statements with respect to the financial condition, results of operations and business of Philips and certain of the plans and objectives of Philips with respect to these items. By their nature, forward-looking statements involve risk and uncertainty because they relate to events and depend on circumstances that will occur in the future and there are many factors that could cause actual results and developments to differ materially from those expressed or implied by these forward-looking statements.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Eli Kammerman
Masimo Investor Relations
Phone: (949) 297-7077
Email: ekammerman@masimo.com
Evan Lamb
Masimo Media Contact
Phone: (949) 396-3376
Email: elamb@masimo.com
Steve Klink
Philips Groups Communications
Phone: +31 6 10888824
Email: steve.klink@philips.com
Vanessa Bruinsma-Kleijkers
Philips Investor Relations
Phone: +31 20 5977447
Email: vanessa.bruinsma-kleijkers@philips.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Adam Mikkelson of Camber Capital Management Joins Masimo’s Board of Directors
Irvine, California – October 27, 2016 – Masimo (NASDAQ: MASI), a global innovator of noninvasive patient monitoring technologies, announced today that Adam Mikkelson, Partner at Camber Capital Management, LLC, has been elected to Masimo’s Board of Directors.
Mr. Mikkelson has been with Camber Capital Management, a healthcare-focused investment fund, since 2007. He has almost 15 years of experience in the healthcare investment arena, focused on identifying and actively monitoring opportunities in both the therapeutic and medical device sectors. He received his B.Sc. in Business Administration from Boston University.
“Adam’s investment experience and deep knowledge of the medical device industry made him an ideal candidate for Masimo’s Board of Directors,” said Joe Kiani, Founder, Chairman, and CEO of Masimo. “His insight into our complex health care system and his standing in the investment community will be invaluable.”
“I’m excited to be joining Masimo’s Board,” said Mr. Mikkelson. “As a healthcare investor, I’ve enjoyed watching Masimo grow over the years via the continued adoption of its innovative monitoring technologies. I hope that I can make positive contributions to an already strong Board to drive long-term value for Masimo and its shareholders.”
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVI®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (PI). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contacts
Investor Contact:
Masimo
Eli Kammerman
Phone: (949) 297-7077
Email: ekammerman@masimo.com
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Study Investigates the Impact of Masimo Continuous SpHb® and PVi® on Anesthesia-related Mortality
Chicago, Illinois – October 22, 2016 – Masimo (NASDAQ: MASI) announced today the findings of an abstract presented at the American Society of Anesthesiologists' (ASA) Annual Meeting in Chicago. In the study, researchers at Hôpital Dupuytren, part of the Centre Hospitalier Universitaire of Limoges, France (CHU Limoges), investigated the clinical utility of noninvasive, continuous hemoglobin (SpHb®) and PVi® (a measure of the dynamic changes in perfusion index that occur during the respiratory cycle), two Masimo rainbow SET™ measurements. The researchers' goal was to determine, at the scale of a whole hospital, improvement in mortality and transfusion needs.1
In the prospective, single-center, observational study, Professor Nathalie Nathan and colleagues reviewed two sets of patients over two eleven-month periods, before (2013) and after (2014) implementation of a clinical algorithm to guide transfusion and fluid administration. Anesthesiologists, nurses, and residents were trained on the implementation of the clinical algorithm. Masimo Radical-7® Pulse CO-Oximeters® were installed in all operating rooms, recovery rooms, and intensive care units. The Radical-7s were connected to Masimo Patient SafetyNet™* for trend data collection. All surgical patients presenting to the hospital were accepted, with these exceptions: EMT, ophthalmology, odontology, radiology, neurosurgery, and patients less than 18 years of age.
The study included 18,867 patients (in the two groups), of whom 3450 underwent SpHb and PVi monitoring via Radical-7. The patients in the monitoring group received vascular filling with crystalloids or blood, according to the clinical algorithm. Demographic, anesthesia, surgical, and transfusion data were collected in electronic medical records. The researchers compared the percentage of patients in the monitored group who received transfusions within the first postoperative 48 hours to the percentage in the non-monitored group. They also compared mortality rates for each group at 30 days and 90 days following surgery.
Using the cox-proportional hazard model, the researchers found that the patients in the group monitored with SpHb and PVi had a 30% reduction in mortality at 30 days and a 25% reduction in mortality at 90 days. The proportion of patients receiving transfusions did not change significantly between the two groups (7.9% in 2013, 8.5% in 2014, p = 0.1323), nor did the number of blood units transfused within 48 hours (3.4 ± 2.7 in 2013, 3.4 ± 2.0 in 2014, p less than 0.05). However, in non-cardiac surgery, patients were transfused sooner in the operative or recovery room (72.9% vs 56.1%, p = 0.0002).
The researchers concluded that "Monitoring SpHb and PVi integrated in a vascular filling algorithm allowed earlier transfusion and reduces mortality at a scale of a whole hospital with different clinical practices (and practitioners) and unselected patients."
"Access to continuous monitoring of Hb levels and fluid responsiveness has changed the way we address blood and fluid management. By lowering inadequate fluid filling at the beginning of anesthesia, we are able to avoid diluting patients inadequately and this data helps us to guide precisely the amount of fluids or blood that must be given to patients on a case by case basis," stated Professor Nathan, Head of the Department of Anesthesiology at CHU Limoges. "Patients are transfused earlier when needed and hypovolemia is precisely treated with crystalloid. These two facts may explain the decrease in mortality at one and three months that we observed in this study. We strongly believe that surgeries of intermediate severity such as hip or knee replacement procedures as well as severe surgery will benefit from this technology. Because it is easy to use, quick to administer, provides continuous data, and does not harm the patient in any way, it is more applicable to common clinical practice."
Joe Kiani, Founder and CEO of Masimo, commented, "We have created technologies that have been shown to save babies' eyesight2, screen for CCHD in newborns3, and reliably monitor patients in post-surgical wards4,5,6, but this is the first time a study has shown that one of our technologies has such a big impact on mortality. Needless to say, we are excited and thank Dr. Nathan for her and her colleagues' research. We look forward to more studies like this that investigate the impact of SpHb and PVi on other patients at other hospitals, and hope to see similar results."
SpHb monitoring may provide additional insight to the directional trend of hemoglobin between invasive blood samplings – when the SpHb trend is stable and the clinician may otherwise think hemoglobin is decreasing; when the SpHb trend is rising and the clinician may otherwise think hemoglobin is not rising fast enough; or when the SpHb trend is decreasing and the clinician may otherwise think hemoglobin is stable. SpHb monitoring, accompanied by laboratory diagnostic testing, may thus help clinicians make more timely and informed decisions, and has been shown to help clinicians provide more timely blood transfusions** and reduce blood transfusions in cases such as neurosurgery and orthopedic surgery.7,8
@MasimoInnovates || #Masimo
*The use of the trademark SafetyNet is under license from University HealthSystem Consortium.
**Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Nathan N et al. Impact of Continuous Perioperative SpHb Monitoring. Proceedings from the 2016 ASA Annual Meeting, Chicago. Abstract #A1103.
2. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
3. de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338.
4. Taenzer AH et al. Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-And-After Concurrence Study. Anesthesiology. 2010; 112(2):282-287.
5. Taenzer AH et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
6. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
7. Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedia Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
8. Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb® and PVi®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb and PVi, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Announces CE Marking of Rad-97™ Pulse CO-Oximeter®
Neuchatel, Switzerland – October 6, 2016 – Masimo (NASDAQ: MASI) announced today the CE marking of the Rad-97™ Pulse CO-Oximeter®. Rad-97 offers the same Measure-through Motion and Low Perfusion™ SET® pulse oximetry and upgradeable rainbow SET™ technology as the Radical-7® Pulse CO-Oximeter – in a versatile standalone monitor configuration.
-
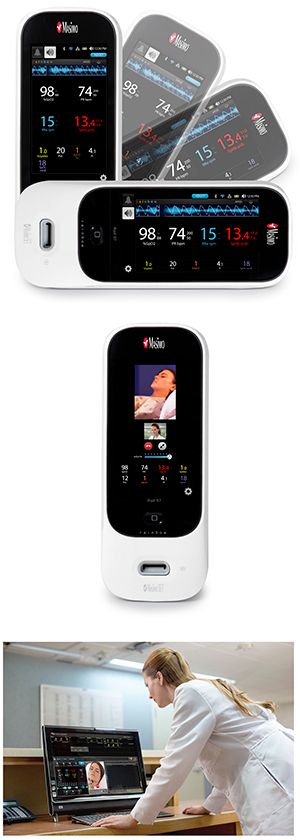
top: Masimo Rad-97™ Pulse CO-Oximeter®
center: Masimo Rad-97 with Camera
bottom: Masimo Patient SafetyNet™ with SafetyNet Surveillance
Rad-97 features a high-resolution 1080p HD color display with user-friendly multi-touch navigation, similar to Root® and Radical-7, allowing clinicians to easily customize the device to best suit their monitoring needs. Users can also rapidly configure the device to accommodate different patient populations using customizable profiles. Rad-97 provides both built-in wireless connectivity, via Wi-Fi and Bluetooth®, and built-in wired options, including Ethernet, USB, and Nurse Call Interface connections. A rechargeable battery lasting seven hours allows Rad-97 to be used in situations where portability or extended operation without access to power are needed.
In select markets outside the United States, Rad-97 will be available with an optional camera, which will be used in conjunction with Masimo Patient SafetyNet™*. The camera includes a high resolution, high-frame rate video feed, as well as audio, to the Patient SafetyNet view-station. The system will be able to use the hospital's existing IT network and to provide additional viewing of images in the same care area. Through Rad-97, it will be possible to connect other devices a patient may be using, such as glucometers and scales, to Patient SafetyNet and to remotely allow for interaction between clinicians and patients or other clinicians.
Like Radical-7, Rad-97 features Measure-through Motion and Low Perfusion pulse oximetry (SpO2), pulse rate (PR), and perfusion index (Pi). Clinicians can add other monitoring solutions such as the rainbow SET measurements total hemoglobin (SpHb®), methemoglobin (SpMet®), acoustic respiration rate (RRa®), carboxyhemoglobin (SpCO®), and oxygen content (SpOC™). In select markets outside the United States, additional parameters such as Oxygen Reserve Index™ (ORi™) and respiration rate from the pleth (RRp™) are available, making Rad-97 the smallest Masimo bedside device currently capable of monitoring the full rainbow SET platform.
"Rad-97 brings our core SET® and rainbow® technologies to a compact new design, which will allow broader applications in many new settings, including low-resource countries and homes," said Joe Kiani, Founder and CEO of Masimo. "We've engineered Rad-97 to take advantage of the latest advances in adaptability and connectivity, packaging Masimo's industry-leading noninvasive technology in a cost-effective monitor that will make it more accessible and useful to clinicians and patients than ever."
ORi and RRp parameters and the camera feature do not have 510(k) clearance and are not available in the U.S.
*The use of the trademark SafetyNet is under license from University HealthSystem Consortium.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo's Rad-97™ Pulse CO-Oximeter®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-97 Pulse CO-Oximeter, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Honors Birthday of Mahatma Gandhi with Global Holiday
Irvine, California – October 2, 2016 – Masimo (NASDAQ: MASI) announced today that in honor of the birthday of Mahatma Gandhi (October 2, 1869), the company will close worldwide and offer an additional paid holiday to employees on Monday, October 3rd.
Mahatma Gandhi, born Mohandas Karamchand Gandhi, is considered the preeminent leader of the Indian independence movement, famous for seeking change, conflict resolution, and agreement through nonviolent protest, often through public fasting. He is still honored today as a global symbol of peace, with his birthday a national holiday in India and celebrated worldwide as the International Day of Nonviolence.
"Masimo has chosen to honor Gandhi's birthday to remind its employees and partners worldwide of the enduring importance of those role models who have helped shape our world for the better. At Masimo, we strive each day to remain true to our promises and responsibilities," said Joe Kiani, Founder and CEO of Masimo. "For us, that includes being thoughtful citizens of the world, striving to improve patient care through our innovative products and technologies. In these difficult times, when injustice, violence, and hatred are never far from the headlines, it's especially important to remember and admire the life of one who so consistently embodied peace, forgiveness, and understanding. Gandhi got what his people wanted and gained his country's independence from British rule through peaceful civil disobedience. I hope all Masimo employees worldwide will use this extra day to take stock of their lives, their goals, and their responsibilities, and to take steps to create a more just and peaceful world."
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Partners with Smile Train to Advance Safe Surgery
Irvine, California – September 30, 2016 – Masimo (NASDAQ: MASI) announced today that it is partnering with Smile Train to help ensure the safety of patients undergoing cleft lip and/or palate surgery in low-resource settings around the world.
Patient monitoring, including the use of pulse oximetry, is a key part of ensuring safe surgical care; however, in lower-resource settings essential monitoring equipment is often unavailable, particularly in the post-operative period.1 Working together, Smile Train and Masimo are helping to remove this barrier by equipping surgical programs in 15 countries in Asia, Africa, Central America and the Middle East with pulse oximeters.
Smile Train is an international organization that leverages the "teach a man to fish" model to train and empower local medical professionals to provide safe, high-quality, comprehensive cleft treatment for children in their own communities. Through Smile Train's local partnerships in 85+ countries, the organization has provided more than 1,000,000 cleft surgeries to date.
Through this partnership, Masimo is providing over 460 Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximeters to Smile Train's nurse training programs and partner hospitals to support improved patient monitoring and safety. Additionally, Masimo and Smile Train will work together to develop additional initiatives to ensure that post-operative pulse oximetry can be scaled across all Smile Train programs.
One of the most common birth defects in the world, a cleft lip and/or palate occurs before a child is born, when the lip and/or roof of the mouth fail to fuse together properly during fetal development. In higher-resource settings virtually all babies with clefts have reconstructive surgery as it is relatively simple, inexpensive, and immediately transformative. This is not the case in lower-resource settings, where, as highlighted by the World Federation of Societies of Anaesthesiologists' SAFE-T Campaign, an estimated 70% of the world's population do not have access to safe and affordable anesthesia and surgical care.1,2 This startling gap in surgical access means the estimated 170,000 children born with clefts in lower-resource settings do not receive surgical treatment and live with difficulties eating, breathing, speaking, and, in the longer term, are less likely to enroll in school or hold jobs due to the stigmas associated with physical defects.3
"Proper surgical monitoring, including essential pulse oximetry, is crucially important to ensuring safe surgical outcomes. Smile Train is excited to partner with Masimo to make their high-performing, Signal Extraction Technology pulse oximeters available at Smile Train partner hospitals as we work together to continue providing safe and quality cleft care to children in need all over the world," said Susannah Schaefer, CEO of Smile Train.
"One of the greatest joys is seeing a smile on the face of a child. Every child on earth deserves to have access to safe surgery, to be treated with dignity and love, and to share their beautiful smiles," said Joe Kiani, Founder and CEO of Masimo. "Partnering with Smile Train is a fundamental part of our commitment to the Sustainable Development Goals and to our belief that no matter where a person lives or where they were born, access to quality healthcare that is dignified and safe is a human right, not a privilege. We are very proud of the work that Smile Train has done to improve surgical safety and access for cleft patients in local communities around the world. We value their commitment to scaling the use of quality pulse oximetry to all programs."
Friday, October 7th is World Smile Day®, on which we are reminded to "Do one act of kindness. Help a person smile!"4 The smile is an international symbol of peace, happiness, and kindness that knows no barriers or boundaries. Masimo and Smile Train are proud to be partnering to bring smiles to the faces of children around the globe, not just on World Smile Day®, but on every day of the year. We encourage everyone to participate in World Smile Day® this year!
References
1. Meara JG et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015 Aug 8;386(9993):569-624.
2. World Federation of Anaesthesiologists (WFSA) Safe Anaesthesia For Everybody – Today "SAFE-T" Campaign, 2016.
http://www.wfsahq.org/get-involved/safe-t.
3. Smile Train Medical Advisory Board.
4. http://www.worldsmileday.com/.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORi™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
About Smile Train
Smile Train is an international children's charity with a sustainable approach to a single, solvable problem: cleft lip and palate. Millions of children in developing countries with untreated clefts live in isolation, but more importantly, have difficulty eating, breathing and speaking. Cleft repair surgery is simple, and the transformation is immediate. Our sustainable model provides training, funding, and resources to empower local doctors in 85+ developing countries to provide 100%-free cleft repair surgery and comprehensive cleft care in their own communities. To learn more, please visit smiletrain.org.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo pulse oximetry. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo pulse oximetry, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Shari Mason
Smile Train
Phone: (212) 689-9199
Email: smason@smiletrain.org
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Foundation Co-Founds United for Oxygen Alliance
Groundbreaking Public-Private Partnership to Increase Access to Oxygen and Pulse Oximetry Launched at Clinton Global Initiative
Irvine, California – September 24, 2016 – Masimo (NASDAQ: MASI) announced today that the Masimo Foundation has co-founded the United for Oxygen Alliance, as part of a Clinton Global Initiative (CGI) commitment. The first-of-its-kind public-private partnership, announced this week at the final CGI Annual Meeting, will seek to expand access to medical oxygen and pulse oximetry for women and children in Ethiopia. Other partners in the Alliance, which has fifteen members in total, include the Bill & Melinda Gates Foundation (BMGF), the United Nations, PATH, Philips, the Pneumonia Innovations Team, Save the Children, UNICEF, and USAID.
Lack of medical oxygen access contributes to the deaths of more than 120,000 young children and thousands of pregnant women, globally, each year. It is estimated that in Ethiopia alone, each year 11,000 women die in pregnancy or during childbirth, 60,000 babies die in their first month, and 30,000 children die from pneumonia.1,2 Many of these deaths could be prevented by better access to medical oxygen and reliable pulse oximetry technology.3 An oxygen access agenda is central to that of the Ethiopian government, which has made it a part of their 2015-2020 Health Sector Transformation Plan and National Newborn and Child Survival Strategy.
United for Oxygen will partner with the Ethiopian government to increase availability of pulse oximetry screenings and oxygen therapy technologies across the country. In addition, it will provide training for local staff; establish sustainable financial solutions for procuring, installing, and maintaining equipment; and promote oxygen access and technologies in the policies and guidelines of Ethiopian health authorities. After the pilot program, the Alliance hopes to roll out similar medical oxygen and pulse oximetry programs in other countries where women and children are particularly at risk.
Leith Greenslade, Co-Chair of the Pneumonia Innovations Team, commented, "United for Oxygen is the first time companies and development agencies have joined forces to support a government to increase access to pulse oximetry and oxygen therapy across an entire African nation – in this case Ethiopia. My hope is that we will demonstrate that not only can pulse oximetry and oxygen prevent many more Ethiopian children from dying of pneumonia, but that health systems that routinely provide pulse oximetry screening and oxygen will be much more effective at driving down death rates from childbirth, unsafe surgery, cardiac arrest and injury."
Masimo, as a global leader in noninvasive monitoring technologies and whose SET® Measure-through Motion and Low Perfusion™ pulse oximetry technology is estimated to be used on more than 100 million patients around the world, will be responsible for improving the availability of pulse oximetry in hospitals and health centers, particularly in maternity and pediatric units. Masimo's role in United for Oxygen will also include developing an efficient, effective health screening model to prevent, detect, and treat pneumonia, as well as collaborating on recommendations for sustaining the program post-deployment through training, education, and maintenance.
Rasa Izadnegahdar, Senior Program Officer on the Pneumonia Team, BMGF, noted, "Masimo is uniquely positioned to provide leadership in the introduction of innovative life-saving devices in partnership with countries as recently exemplified by the Oxygen Roadmap of the Ethiopia Federal Ministry of Health."
"This cannot be overstated: regardless of where you live or were born, you deserve access to quality healthcare – including the life-saving benefits of reliable pulse oximetry and oxygen therapy," said Joe Kiani, Founder and CEO of Masimo. "Masimo continues to champion systematically safe, dignified health care for all, and we are proud for the Masimo Foundation to be one of the founding members of United for Oxygen. We look forward to collaborating further on this important project."
References
1. Kuti BP et al. Determinants of oxygen therapy in childhood pneumonia in a resource-constrained region. ISRN Pediatr. 2013 Jun 2;2013:435976.
2. Rudan I, et al. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008 May; 86(5): 408–416.
3. Floyd, et al. Evaluating the Impact of pulse oximetry on childhood pneumonia mortality in resource-poor settings. Nature. 528, S53-S59 (3 December 2015).
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Study on Abdominal Surgical Patients Investigates Clinical Utility of Masimo PVi®
Irvine, California – September 15, 2016 – Masimo (NASDAQ: MASI) announced today the findings of a study recently presented at the World Congress of Anesthesiologists (WCA) in Hong Kong. In the study, researchers at Cairo University in Egypt investigated the clinical utility of a Masimo SET® noninvasive, continuous measurement, PVi®.
In the prospective study of 60 adult patients undergoing major abdominal surgery, Drs. Hafez and Helmy of Cairo University evaluated the performance of Masimo PVi as a non-invasive guide to fluid optimization, as compared to Transesophageal Doppler. Patients were divided into fluid responders and non-responders, based upon the change in stroke volume due to a bolus response. The baseline value of PVi was significantly higher (p < 0.05) in responders compared to non-responders. The authors also noted that a baseline PVi cutoff value of 11 had 96.67% sensitivity and 33.33% specificity for predicting greater than 10% stroke volume increase. They concluded that PVi is "an efficient predictor of fluid responsiveness...in patients undergoing elective major abdominal surgery."1
PVi is a measure of the dynamic changes in perfusion index (Pi) that occur during the respiratory cycle. In clinical studies, PVi has been shown to help clinicians assess fluid responsiveness in mechanically ventilated patients under general anesthesia during surgery,2,3,4,5 in the ICU in both adults and children,6,7 and in septic patients in the early stages of shock in the emergency department.8 Another study used PVi as part of goal-directed therapy for patients in an enhanced recovery after surgery (ERAS) program who underwent colorectal surgery; the program led to significant reductions in lengths of stay, costs, surgical site infections, fluid administered, as well as improvement in patient satisfaction.9
References
1. Hafez, Helmy. Evaluation of Plethysmographic variation indices for assessing fluid responsiveness in major operations using Masimo Radical-7 Pulse CO-Oximeter. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR371.
2. Cannesson M. et al. Br J Anaesth. 2008;101(2):200-6.
3. Zimmermann M., Feibicke T., Keyl C., Prasser C., Moritz S., Graf B.M., Wiesenack C. Eur J Anaesthesiol. 2010 Jun;27(6):555-61.
4. Fu Q., Mi W.D., Zhang H. Biosci Trends. 2012 Feb;6(1):38-43.
5. Haas S., Trepte C., Hinteregger M., Fahje R., Sill B., Herich L., Reuter D.A. J Anesth. 2012 Oct;26(5):696-701.
6. Loupec T. et al. Crit Care Med. 2011;39(2):294-299.
7. Byon H et al. BJA. 2012 December; DOI 10.1093/bja/aes467.
8. Feissel M et al. J Crit Care. 2013 May 14
9. Thiele RH, Rea KM, Turrentine FE, Friel CM, Hassinger TE, Goudreau BJ, Umapathi BA, Kron IL, Sawyer RG, Hedrick TL. Standardization of Care: Impact of an Enhanced Recovery Protocol on Length of Stay, Complications, and Direct Costs after Colorectal Surgery. Journal of the American College of Surgeons (2015). doi: 10.1016/j.jamcollsurg.2014.12.042.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo PVi®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo PVi, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


New Study Finds Continuous, Noninvasive Hemoglobin Monitoring Using Masimo SpHb® May Reduce Intraoperative Red Blood Cell Transfusion
Irvine, California – September 9, 2016 – Masimo (NASDAQ: MASI) announced today announced today that in a new study recently presented at the World Congress of Anesthesiologists (WCA) in Hong Kong, researchers concluded that continuous and noninvasive hemoglobin monitoring, using Masimo SpHb®, may reduce excessive intraoperative red blood cell (RBC) transfusion.1
In a retrospective review of 371 patients who underwent intraoperative RBC transfusions between 2012 and 2014 at Fukushima Medical University in Japan, Dr. Imaizumi and colleagues compared 94 patients who had noninvasive hemoglobin measurements to 277 patients who did not (the control group). The total transfusion volumes and transfusion volume per 1 g of blood loss were determined for each group.
Comparing the groups, researchers noted that "a significantly lower mean RBC transfusion volume per 1 g of blood loss was observed in the SpHb group compared with the [control] group (SpHb group, 0.9 ± 1.0 ml/g blood loss vs [control] group, 2.4 ± 5.9 ml/g blood loss, p < 0.01)." They also observed that there was "no significant difference...in the average RBC transfusion volume (SpHb group, 815 ± 819 ml vs. [control] group, 785 ± 773 ml, p=0.75), or the preoperative hemoglobin concentration (SpHb group, 10.4 ± 1.9 g/dL vs. [control] group, 10.2 ± 2.4 g/dL, p=0.27) between the groups." According to results from this abstract, the authors concluded that "SpHb measurements are associated with reducing excessive intraoperative RBC transfusion."
"This is the third study, published by different researchers on three continents (US2, Egypt3, and now Japan1) that has shown that in addition to other clinical tools, SpHb may be used to help clinicians make informed transfusion decisions during different types of surgery*," stated Dr. Steven Barker, Ph.D., M.D., Chief Science Officer, Masimo.
SpHb monitoring may provide additional insight to the directional trend of hemoglobin between invasive blood samplings – when the SpHb trend is stable and the clinician may otherwise think hemoglobin is decreasing; when the SpHb trend is rising and the clinician may otherwise think hemoglobin is not rising fast enough; or when the SpHb trend is decreasing and the clinician may otherwise think hemoglobin is stable. SpHb with laboratory diagnostic test may thus help clinicians make more timely and informed decisions, and has been shown to help clinicians provide more timely blood transfusions* and reduce blood transfusions in cases such as neurosurgery and orthopedic surgery.2,3
Last week Masimo announced that in response to the Zika virus and its potential impact on the availability of blood products, Masimo has created a special program to dramatically reduce the cost of and increase access to Masimo's SpHb solutions, as SpHb has been shown to help clinicians reduce blood transfusions in both low and high blood loss surgery. This special program will be available wherever the blood supply is affected by the Zika virus.
*Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgement considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
2. Ehrenfeld JM et al. J Blood Disorders Transf. 2014. 5:9.
3. Awada WN et al. J Clin Monit Comput. 2015 Feb 4.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®and the availability of Masimo's special program for SpHb solutions. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo MightySat™ is Available at Apple.com and Select Apple Retail Stores in the US and Canada
Apple Health App Integrates MightySat Data
Irvine, California – September 6, 2016 – Masimo (NASDAQ: MASI) announced today that MightySat™, a fingertip pulse oximeter designed for general wellness and health applications including sports, fitness, and relaxation management, is now available at Apple.com and select Apple retail stores in the US and Canada.
-
Masimo MightySat™ and Personal Health App
Masimo pulse oximeters, the leading brand in hospitals, provide accurate measurements when other pulse oximeters fail. By using a revolutionary invention called Signal Extraction Technology® (SET®), which allows oxygen saturation and pulse rate to be measured through challenging conditions including movement and low blood flow, the same high performance Masimo SET® technology used in hospitals is now available to Apple customers with MightySat.
The MightySat provides the following noninvasive measurements:
- Oxygen Saturation (SpO2)
- Pulse Rate (PR)
- Perfusion Index (Pi)
-
Masimo SET® Accuracy Compared to That of 19 Other Hospital-Grade Pulse Oximeters
In a study of 70 healthy volunteers during conditions of movement and low blood flow, Masimo SET® was the most accurate pulse oximeter when compared to 19 other hospital-grade pulse oximeters.1 SpO2 accuracy was defined as being within 7% of the reference value and pulse rate accuracy as being within 10%. Masimo SET® SpO2 was accurate 94% of the time while on average, the 19 other hospital-grade pulse oximeters were accurate only 60% of the time, with the least accurate only displaying accurate SpO2 27% of the time. Masimo SET® pulse rate was accurate 85% of the time while on average, the 19 other hospital-grade pulse oximeters were accurate only 27% of the time, with the least accurate only displaying accurate pulse rate 5% of the time.
Leading athletes and sports teams are increasingly using MightySat. "The reliable measurements of oxygen saturation and pulse rate from Masimo pulse oximeters were an important part of my training and recovery regimen to achieve peak athletic performance," said Dotsie Bausch, 2012 Olympic medalist in cycling. "I trust MightySat to accurately track and trend my data on a daily basis and help me achieve optimal health, wellness, and fitness."
MightySat users can display and track their data on a compatible iOS® device via the free Masimo Personal Health app available in the App Store. With user permission MightySat securely stores and trends measurements displayed in the Masimo Personal Health app in Apple's Health app. With the most recent update, the Masimo Personal Health app also now features same-screen trending for all MightySat measurements.
The MightySat fingertip pulse oximeter is intended for general wellness and health applications. For medical applications, Masimo offers the MightySat Rx, which is available to medical professionals.
Reference
1. Barker S.J. Anesth Analg. 2002 Oct;95(4):967-72.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo MightySat™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our expectations regarding how retail customers will react to and benefit from the Masimo MightySat; risks related to our assumptions regarding the repeatability of study results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo MightySat, contribute to positive outcomes; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Announces Amendment to Nellcor Royalty Agreement
Irvine, California – September 2, 2016 – Masimo (NASDAQ: MASI) today announced an amendment to the existing settlement agreement initially reached between Masimo and Nellcor in January 2006. Under the terms of the amendment, Medtronic will continue to pay Masimo a royalty of 7.75% for its current pulse oximetry products sold in the United States through October 6, 2018, after which no further royalties will be due under the agreement. For more information, please see the related Form 8-K filed with the Securities and Exchange Commission on September 2, 2016.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Investor Contact:
Eli Kammerman
Masimo
Phone: (949) 297-7077
Email: ekammerman@masimo.com
Media Contact:
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Announces Four-Year Partnership with World Federation of Societies of Anaesthesiologists (WFSA)
Joint Initiative, "Safe Anaesthesia – ASAP," Is to Improve Anesthesia Care in Low Resource Countries
Irvine, California and Hong Kong, China – August 29, 2016 – Masimo (NASDAQ: MASI) announced Sunday at the World Congress of Anaesthesiologists (WCA) in Hong Kong that it has become the first Global Impact Partner of the World Federation of Societies of Anaesthesiologists (WFSA). The four-year partnership, "Safe Anaesthesia – ASAP" (Anesthesia Safety Action Plan) will initially identify one country with poor access to safe anesthesia, and will work toward implementing programs and training designed to improve anesthesia care and safe surgery outcomes.
In 2015, the Lancet Commission on Global Surgery reported that approximately 5 billion people – the majority of the world's population – do not have access to safe, affordable anesthesia and surgical care, and estimated that 16.9 million die annually as a result.1 These deaths are attributable to a lack of infrastructure, essential monitoring equipment, and drugs, as well as a shortage of adequately trained healthcare workers.1
As part of the first phase of the joint project between the WFSA and Masimo, a high burden country will be identified as the ASAP's focus. Key to the project's success will be bringing together all stakeholders, including the government, so that infrastructure gaps can be identified, needs assessed, and the ASAP implemented. Implementation will involve a combination of training courses and other educational materials, distribution of essential drugs, and installation of and training on medical equipment. Training, monitoring, and project assessment will be ongoing throughout the four years, with an emphasis on ensuring that the improvements are sustainable.
Lifebox, an NGO (not-for-profit, non-governmental organization) dedicated to improving safe surgery, will also be assisting with training support. Kristine Stave, Chief Operating Officer of Lifebox, commented, "Lifebox works with colleagues on the frontline of the global surgical safety crisis to support long term improvement in the safety and quality of anesthesia care. We know firsthand the challenges of environment, equipment, education – and we also know the extraordinary capacity for positive change that the best partnerships can bring. We're proud to be a part of this urgent and historic undertaking between the WFSA and Masimo, and to be delivering our shared goal of making anesthesia safer for millions of patients worldwide."
WFSA has been uniting anesthesiologists globally for more than 60 years. With a network of hundreds of thousands of anesthesiologists in more than 150 countries, WFSA is well positioned to deliver programs that facilitate learning, and promote the highest standard of anesthesia care globally. Masimo, whose clinically leading SET® pulse oximetry is used to monitor over 100 million patients across the world, has made a commitment to become a WFSA Global Impact Partner in 2016. Together, WFSA and Masimo created the ASAP action plan, which it is hoped will become a model for high burden countries to improve anesthesia care and safe surgery outcomes.
Dr. David Wilkinson, President of the WFSA, stated, "The WFSA is delighted to announce Masimo as our first Global Impact Partner. We work together to improve patient care and access to safe anesthesia around the world."
Dr Adrian Gelb, Chair of the WFSA's Safety and Quality Committee and Professor of Anaesthesia and Perioperative Care at the UCSF School of Medicine, added, "It is essential that we make safe and affordable anesthesia and surgical care available to every patient in every country. Investment in national anesthesia plans, together with key stakeholders, will ensure that specific country needs are met most appropriately in order to make the biggest impact."
"At Masimo, we believe that regardless of where you live or where you were born, access to quality healthcare that is dignified and safe – including safe, effective anesthesia and surgery – is a human right, not a privilege," said Joe Kiani, Founder and CEO of Masimo. "We are delighted to be the first partner with the WFSA in bringing much-needed help, with Safe Anaesthesia – ASAP, to areas of the world blighted by needless deaths."
Mr. Kiani added: "The new millennium goal is no longer to provide all of our people access to health care, but to provide all of our people access to health care that is systematically safe and dignified."
References
1. Meara JG, Leather AJ, Hagander L et al. Global Surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Surgery. 2015 Jul;158(1):3-6.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential success of the ASAP. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Announces a Special Worldwide Program for Total Hemoglobin (SpHb®) Monitoring for Areas Affected by the Zika Virus
Irvine, California – August 29, 2016 – Masimo (NASDAQ: MASI) announced today that in response to the Zika virus and its potential impact on the availability of blood products, Masimo is creating a special program to dramatically reduce the cost of and increase access to Masimo's continuous hemoglobin monitoring (SpHb®) solutions, shown to help clinicians reduce blood transfusions in both low and high blood loss surgery. This special program will be available wherever the blood supply is affected by the Zika virus.
Masimo's special program will allow clinicians and institutions in areas affected by the Zika virus to obtain a 50% discount off the list price of noninvasive SpHb sensors, and in addition, will make a limited number of loaner SpHb monitoring devices available at no cost during this special program.
Joe Kiani, Founder and CEO of Masimo, stated, "Because of how the Zika virus is transmitted, the FDA has recently recommended that the existing blood supply be tested for the virus, in addition to adding Zika screening for new blood donations. While this additional testing is necessary, it may constrain supplies of usable blood products while also increasing the cost of blood for a period of time. Masimo wants to do its part to help clinicians and healthcare facilities mitigate these issues by dramatically reducing the price of and increasing access to our SpHb monitoring solutions, which permit continuous, real-time visibility to changes in hemoglobin levels to help clinicians facilitate more informed and timely transfusion decisions. Independent, published studies completed by different researchers on three continents (US1, Egypt2, Japan3) have shown that SpHb may help clinicians reduce blood transfusions during different types of surgery*."
"We believe the use of continuous SpHb monitoring can help reduce demand for donated blood and the risk posed by blood donated before the additional testing, which may be infected," Mr. Kiani added. "In addition, Masimo will expand its ongoing efforts to increase awareness of the transfusion reduction benefits of the multidisciplinary pillars of Patient Blood Management, including the importance of pre-operative screening and therapies to safely increase patients' hemoglobin levels before elective surgery4."
*Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgement considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Ehrenfeld JM et al. J Blood Disorders Transf. 2014. 5:9.
2. Awada WN et al. J Clin Monit Comput. 2015 Feb 4.
3. Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
4. Meybohm P et al. Patient Blood Management is Associated With a Substantial Reduction of Red Blood Cell Utilization and Safe for Patient's Outcome. Ann Surg. 2016;264:203-211.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Announces CE Marking for RAS-45 Sensor for rainbow Acoustic Monitoring® of Respiration Rate
Neuchatel, Switzerland – August 18, 2016 – Masimo (NASDAQ: MASI) announced today the CE Marking of RAS-45, a single-use adult and pediatric acoustic respiration sensor for rainbow Acoustic Monitoring® (RAM™) of respiration rate (RRa®).
-
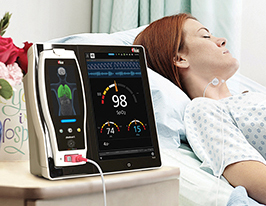
Masimo Root® with rainbow Acoustic Monitoring® (RAM™) of Respiration Rate (RRa®) with RAS-45 Sensor
Continuous monitoring of respiration rate is especially important for post-surgical patients receiving patient-controlled analgesia for pain management. The Anesthesia Patient Safety Foundation (APSF) and The Joint Commission recommend continuous oxygenation and ventilation monitoring for all patients receiving opioid-based pain medications.1,2 RAM noninvasively and continuously measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer, such as Masimo's RAS-125c and now RAS-45, that is applied to the patient's neck. Using acoustic signal processing that leverages Masimo's breakthrough Signal Extraction Technology (SET®), the respiratory signal is separated and processed to display continuous acoustic respiration rate (RRa). RRa has been shown to be accurate, easy-to-use, and reliable, and to enhance patient compliance.3,4 RRa may facilitate earlier detection of respiratory compromise and patient distress, offering a breakthrough in patient safety for post-surgical patients and for procedures requiring conscious sedation.4
-

Masimo RAS-45 Sensor
RAS-45 is designed to facilitate placement on and improve attachment to the neck, but with a smaller adhesive profile than the RAS-125c. It is flexible and uses a transparent adhesive. Like the RAS-125c, it operates with Masimo MX technology boards to measure RRa and display the acoustic respiration wave form. RAS-45 maintains the same performance parameters, range, and accuracy specifications as RAS-125c. Both sensors are for patients who weigh more than 10 kg.
Joe Kiani, Founder and CEO of Masimo, commented, "RAM harnesses the power of our breakthrough signal processing technology, using Masimo SET® and rainbow® technologies, and applies those achievements to a respiratory measurement derived from the sound of breathing. Studies have found that RAM RRa is more sensitive to detecting respiratory pause5 and yet easier for clinicians and patients to use."3
References
1 Stoelting, RK et al. APSF newsletter. 2011. www.apsf.org.
2 The Joint Commission Sentinel Event Alert. Issue 49, August 8, 2012. www.jointcomission.org.
3 Macknet MR et al. Anesthesiology. 2007;107:A84 (abstract).
4 Goudra BG et al. Open J Anesthesiol. 2013;3:74-79.
5 Ramsay MAE et al. Anesthesia-analgesia. 2013:117:1.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo RAM™, RRa®, and RAS-45. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo RAM, RRa, and RAS-45, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


New Article Showcases an Effective Surveillance System for General Care Units Using Masimo Patient SafetyNet™
Irvine, California – August 15, 2016 – Masimo (NASDAQ: MASI) announced today that a new article published in The Joint Commission Journal on Quality and Patient Safety reports on the results of an expansion of the use of Masimo Patient SafetyNet™* to more than 200 general floor beds at Dartmouth-Hitchcock Medical Center (D-H) in Lebanon, New Hamphire.1 Patient SafetyNet is a remote monitoring and clinician notification system that works in conjunction with a variety of bedside monitors, such as Masimo Root® with Radical-7® and Root with Radius-7®, powered by Masimo SET® pulse oximetry – the first Measure-through Motion and Low Perfusion™ pulse oximetry and the most accurate and reliable pulse oximeter in such conditions according to over 50 studies.2
Masimo Patient SafetyNet was first implemented in the 36-bed orthopedic unit at D-H in December 2007, as part of a surveillance strategy supporting patient safety improvement. Clinicians reported that after 11 months, rescue events had been reduced by 65% and intensive care unit transfers by 48%, and calculated annual cost savings of $1,480,000.3,4 They also announced that they had experienced zero preventable deaths or instances of irreversible seizure brain damage due to opioids since installation.4 As a result of the positive outcomes of the pilot unit implementation, D-H expanded this continuous patient monitoring approach, including the use of Patient SafetyNet, to remaining post-surgical ward units in February 2009, to medicine ward units in April 2010, and to the pediatric ward unit in February 2012. Patients in more than 200 beds are now covered by D-H's patient surveillance system. As the authors note, "The original success in terms of patient outcomes and enthusiastic endorsement by nurses prompted request from other units for the system and led to the rapid spread of the system across the institution." The improvements first seen in the pilot unit – in addition to the dramatic reductions in rescue events and unplanned transfers, approximately two alarms per patient per 12-hour nursing shift, and resolution of more than 85% of all alarm conditions within 30 seconds and more than 99% before escalation was triggered – have been "sustained over time in spite of increasing patient acuity (up 20% from 2010 to 2015) and unit occupancy (93% in 2015)."
Much of the initiative's success is due to D-H's development of a robust general care alarm management strategy, including the implementation of Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry. As the authors note, "device-level characteristics such as measurement reliability and alarm annunciation" are critical. A systematic approach to alarm management is needed because of the growing problem of "'alarm fatigue' – the growing desensitization of health care providers to alarms" as a result of the "growing number of monitoring devices, combined with suboptimal patient monitoring and alarm management strategies." False and/or nonactionable alarms may occur as much as 90% of the time, according to one study5; another, involving the Philips HP Merlin M1094 monitor, found that 77% were either not recognized or ignored6; a third, involving Nellcor pulse oximetry, found an actual risk for patients in only 3% of alarm states, and that anesthesiologists have been shown to disable alarms because of high false alarm rates.7
D-H attempted to optimize several key elements of an effective alarm management strategy: 1) static alarm settings – setting thresholds based on patient groups to reduce nuisance alarms; 2) alarm delays – introducing a delay of 15 seconds before an alarm sounds, as "Many changes in physiologic parameters are brief and self-correcting"; 3) alarm threshold adjustments – adapting to "physiologic variation among different patients" by adjusting alarms based on a three-tiered system of the static defaults, independent nurse adjustments, and provider-ordered individualized settings; and 4) alarm announcement – having the alarm sent to the nurse in charge of the patient via remote pager and allowing customization of when and how alarms are escalated to additional clinicians, by which practice alarm exposure has now been reduced by almost 90%.
Important to the success of the D-H alarm management strategy was the selection of Masimo SET® and Patient SafetyNet architecture, as part of D-H's patient surveillance system. As the authors note, a fundamental factor affecting operation of and response to clinical monitor alarms is "device-level characteristics such as measurement reliability and alarm annunciation." As the initiative's centrally-monitored parameter they notably chose oxygen saturation (SpO2), measured using Masimo SET® pulse oximetry, which has been shown to reduce false alarms by over 95% and increase true alarm detection to over 97%, even during motion and low perfusion.8 Regarding the choice of remote monitoring system, the authors note that "A previously installed multiparameter monitoring system on the pediatric unit was largely rejected by the staff because of alarm system issues, such as high false alarm rates, lack of directed notification, and ambiguous alarm indicators. The alarm rate [with Masimo Patient SafetyNet] was 75% lower than the alarm rate of the previous system immediately following implementation of [Masimo Patient SafetyNet], and 100% of nursing staff survey respondents were in favor of continuing use of [Masimo Patient SafetyNet]. The staff credited the robust alarm management strategy and leadership rounding with the improved system performance and high level of staff adoption."
"Dartmouth-Hitchcock provides a compelling example of the benefits that a robust patient monitoring and surveillance system, coupled with a carefully executed strategy, can reap," said Joe Kiani, Founder and CEO of Masimo. "It is estimated that as many as 50,000 patients die each year due to failure to rescue patients in the general ward.9 Any hospital that wants to eliminate general ward preventable deaths should read both this article and Dartmouth-Hitchcock's original study, 'Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: A before-and-after concurrence study,' published in 2010.3 Together, they provide an excellent guide to designing, implementing, and maintaining a successful hospital-wide continuous monitoring system, as well as showcasing the innovative practices that have arisen out of their focus on alarm management. Dartmouth-Hitchcock's success story, laid out in a well-executed plan and follow on analysis over nearly a ten-year period, sets a powerful precedent for other medical facilities: it's time for all hospitals to develop hospital-wide alarm management strategies and as part of that, to implement Patient SafetyNet – to help save money and save lives."
References
1. McGrath, S.P., Taenzer, A.H., Karon, N, Blike, G. "Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation." The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
2. All published clinical studies on Masimo products can be found at http://www.masimo.com/cpub/clinical-evidence.htm.
3. Taenzer A.H., Pyke J.B., McGrath S.P., Blike G.T. Anesthesiology. 2010 Feb;112(2):282-7.
4. Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring - The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online.
5. Imhoff M, Kuhls S. Alarm algorithms in critical care monitoring. Anesth Analg. 2006;102:1525-1537.
6. Gorges M, et al. Improving alarm performance in the medical intensive care unit using delays and clinical context. Anesth Analg. 2009;108:1546-1552.
7. Kestin IG, Miller BR, Lockhart CH. Auditory alarms during anesthesia monitoring. Anesthesiology. 1988;69:106-109.
8. Shah N et al. J Clin Anesth 2012 Aug;24(5):385-91.
9. HealthGrades Quality Study. Patient Safety in American Hospitals (July 2004).
*The use of the trademark SafetyNet is under license from University HealthSystem Consortium.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Patient SafetyNet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Evan Lamb
Masimo
Phone: (949) 396-3376
Email: elamb@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Announces CE Marking for Pediatric O3™ Regional Oximetry
Neuchatel, Switzerland – August 4, 2016 – Masimo (NASDAQ: MASI) announced today the CE marking for the pediatric indication for O3™ regional oximetry with the O3 pediatric sensor. Regional oximetry, also referred to as tissue or cerebral oximetry, helps clinicians monitor cerebral oxygenation.
-
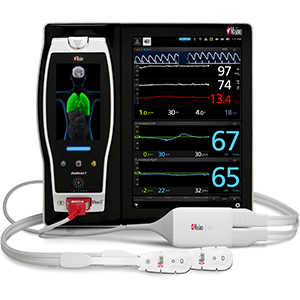
Masimo Root® with O3™ Regional Oximetry with Pediatric Sensor
O3 regional oximetry uses near-infrared spectroscopy (NIRS) to continuously monitor absolute and trended regional tissue oxygen saturation (rSO2) in the cerebral region. Early detection and correction of imbalances in oxygen delivery to the brain and vital organs are important tools in helping patients avoid postoperative morbidity and adverse outcomes.1 With the release of the O3 pediatric sensor, O3 regional oximetry monitoring of rSO2 is now available to pediatric patients weighing less than 40 kg (88 lbs).
"O3 regional oximetry provides access to valuable data about cerebral oxygen saturation, and studies have shown that the risks of cerebral desaturations include neurological injury2,3, increased length of hospital stays3, increased time on mechanical ventilation4, and other adverse outcomes5," said Joe Kiani, Founder and CEO of Masimo. "With adult trend accuracy of 3% and absolute accuracy of 4% without controlling CO2, and trend accuracy of 3% in pediatric patients6, Masimo O3 should help clinicians build a better picture of brain oxygenation – and hopefully better outcomes for all of their patients, including pediatric ones."
Masimo O3 regional oximetry and SedLine® brain function monitoring are both available on a single platform, Masimo Root® – opening up a path to better understanding of the brain.
O3 regional oximetry for use with adults weighing 40 kg (88 lbs) or greater has received FDA 510(k) clearance. O3 regional oximetry for use with pediatric patients weighing less than 40 kg (88 lbs) has not received FDA 510(k) clearance; the O3 pediatric sensor is not currently for sale in the United States.
References
1. Booth, Dukatz, Ausman, and Wider. "Cerebral and somatic venous oximetry in adults and infants." Surg. Neurol Int. 2010; 1: 75.
2. Colak Z, Borojevic M, Bogovic A, Ivancan V, Biocina B, Majeric-Kogler V. Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: a randomized, prospective study. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2015 Mar;47(3):447-54.
3. Slater JP, Guarino T, Stack J, et al. Cerebral Oxygen Desaturation Predicts Cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87:36-45.
4. Goldman S, Sutter F, Ferdinand F, Trace C. Optimizing intraoperative cerebral oxygen delivery using noninvasive cerebral oximetry decreases the incidence of stroke for cardiac surgical patients. The heart surgery forum. 2004;7(5):E376-381.
5. Deschamps A, Lambert J, Couture P, et al. Reversal of decreases in cerebral saturation in high-risk cardiac surgery. Journal of cardiothoracic and vascular anesthesia. 2013;27(6):1260-1266.
6. Masimo data on file.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVI®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo O3™ Regional Oximetry. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo O3™ Regional Oximetry, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.



Leading Ohio Critical Access Hospital Adopts Masimo Root® with Radius-7® , Radical-7® , and Patient SafetyNet™ to Create Hospital-Wide Wireless Monitoring and Clinician Notification System
Geneva, Ohio and Irvine, California – July 28, 2016 – Masimo (NASDAQ: MASI) announced today that University Hospitals Geneva Medical Center (UH Geneva), a Critical Access Hospital in Geneva, Ohio, is building a hospital-wide wireless monitoring and clinician notification system using Masimo Patient SafetyNet™* in conjunction with Masimo Root® with Radius-7® and Masimo Radical-7®. UH Geneva is part of University Hospitals, based in Cleveland, Ohio.
Masimo Patient SafetyNet is a supplemental system that enables information from bedside and tetherless wearable monitors to be accessible from remote locations and relays alarm notifications to clinicians. Used with Root, Patient SafetyNet allows hospitals to automate the transfer of patient vital signs, including temperature and blood pressure, to patients' medical records, which may help improve nursing workflows. Root, available with Radical-7 or Radius-7, provides monitoring of Masimo rainbow SET™ parameters such as oxygen saturation (SpO2) and total hemoglobin (SpHb), and is expandable to monitor additional parameters, such as respiration rate from RAM™ (rainbow® acoustic monitoring). Radius-7 provides continuous tetherless wearable monitoring of FDA-cleared rainbow SET™ parameters so that patients can have freedom of movement while being monitored – and studies have shown that patient mobility is a key factor in more rapid patient recovery.1,2 Monitoring parameters from Radical-7 or Radius-7 are sent to Patient SafetyNet through Root, allowing for hospital-wide remote monitoring and clinician notification.
UH Geneva is part of the University Hospitals system, which employs over 26,000 physicians and employees in Northeast Ohio, and whose flagship academic medical center, UH Case Medical Center, is the primary affiliate of Case Western Reserve University School of Medicine. UH Geneva has been using Masimo SET® pulse oximetry for several years. Along with implementing such parameters as RAM acoustic respiration rate, they are now adopting Root with Radius-7, Radical-7, and Patient SafetyNet to create a hospital-wide wireless monitoring solution, which will include remote caregiver notification.
Thomas P. Knowles, MHA, RRT, RPSGT, Manager of Respiratory Therapy and the Center for Advanced Sleep Medicine at UH Geneva, a key stakeholder in implementing the system, commented, "I've always believed in Masimo's technology and felt it was important for us to expand our patient monitoring practices. Patient SafetyNet will provide tremendous benefits."
"Patient safety is extremely important to us," said Dr. Amitabh Goel, Chief Medical Officer at UH Geneva Medical Center. "The addition of Patient SafetyNet, which will work hand-in-hand with Root, Radical-7, and Radius-7, helps ensure our doctors and nurses always have the data they need, when they need it. We're also looking forward to integrating Patient SafetyNet with our EMR system, which will automate many administrative duties and free up our caregivers to focus on the enhanced patient experience we strive for."
"UH Geneva provides a compelling example of the standard of care that more and more community hospitals are adopting," said Joe Kiani, Founder and CEO of Masimo. "Powerful centralized monitoring and surveillance, such as those provided by Patient SafetyNet, fueled by industry-leading rainbow SET™ and innovative devices like Root with Radius-7, are key to patient monitoring, and it's important, and gratifying, that hospitals like UH Geneva recognize this. We look forward to deepening our collaboration with UH Geneva."
References
1. Needham D, Korupolu R, Zanni J, Pradhan P, Colantuoni E, Palmer J, Brower R, Fan E. "Early Physical Medicine and Rehabilitation for Patients With Acute Respiratory Failure: A Quality Improvement Project." Archives of Physical Medicine and Rehabilitation. Vol 91, Issue 4, PP 536–542, April 2010.
2. Ronnenbaum J, Weir J, Hilsabeck T. Earlier mobilization decreases length of stay in the intensive care unit. J Acute Care Phys Ther. 2012;3(2):204-210.
*The use of the trademark SafetyNet is under license from University HealthSystem Consortium.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
About University Hospitals
Founded in May 1866, University Hospitals serves the needs of patients through an integrated network of 18 hospitals, more than 40 outpatient health centers and primary care physician offices in 15 counties throughout Northeast Ohio. At the core of our $4 billion health system is University Hospitals Case Medical Center, ranked among America's best hospitals by U.S. News & World Report. The primary affiliate of Case Western Reserve University School of Medicine, UH Case Medical Center is home to some of the most prestigious clinical and research programs in the nation, including cancer, pediatrics, women's health, orthopedics, radiology, neuroscience, cardiology and cardiovascular surgery, digestive health, transplantation and genetics. Its main campus includes UH Rainbow Babies & Children's Hospital, ranked among the top children's hospitals in the nation; UH MacDonald Women's Hospital, Ohio's only hospital for women; and UH Seidman Cancer Center, part of the NCI-designated Case Comprehensive Cancer Center at Case Western Reserve University. UH is the second largest employer in Northeast Ohio with 26,000 employees. For more information, go to www.UHhospitals.org.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Radical-7®, Root® with Radius-7®, and Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Radical-7, Root with Radius-7, and Patient SafetyNet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
University Hospitals
Dan Bomeli
Phone: (216) 767-8548
Email: daniel.bomeli@UHhospitals.org
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


New Study Evaluates Masimo PVi® as a Predictor of Fluid Responsiveness in Patients Undergoing Liver Transplantation
Irvine, California – July 21, 2016 – Masimo (NASDAQ: MASI) announced today that a new study of patients undergoing liver transplantation evaluated the relationship of PVi® to right ventricular end-diastolic volume (RVEDVI) and concluded that PVi provided a "reliable estimate of [cardiac] preload status and may be a useful predictor of fluid responsiveness."1 PVi measures the dynamic changes in perfusion index that occur during one or more complete respiratory cycles, using the Masimo pulse oximetry plethsymographic waveform.
Because of hemodynamic instability during orthotopic liver transplantation (OLT), estimating cardiac preload to optimize fluid management is essential. There are several methods for estimating cardiac preload. Two conventional static measurements of cardiac filling pressure are pulmonary artery occlusion pressure (PAOP) and central venous pressure (CVP). The standard invasive method is RVEDVI, obtained from thermodilution using a pulmonary artery catheter (PAC).
In the prospective study, published in Transplantation Proceedings and conducted at Chang Gung Memorial Hospital in Taiwan, Dr. H.-C. Lee and colleagues compared methods of estimating cardiac preload, with the aim of evaluating the relationship between a dynamic hemodynamic parameter, PVi (derived from pulse oximetry), and RVEDVI (using a PAC), as well as comparing PVi to the static measures CVP and PAOP. They measured the four hemodynamic parameters–CVP, PAOP, RVEDVI, and PVi–on 18 patients undergoing OLT at 10 defined time points before, during, and at the conclusion of surgery.
Analyzing the 180 measurements from each of the parameters, the Receiver Operating Characteristic (ROC) determined in this study to distinguish different thresholds of RVEDVI showed an area under the curve (AUC) of 0.702 for CVP, 0.748 for PAOP, and 0.762 for PVi.
The researchers concluded that "PVi may serve as a reliable estimate of cardiac preload status in patients undergoing OLT, explicitly, higher PVi values correlated with lower RVEDVI values," so that "an increase in ventricular preload status could be inferred from a decrease in PVi during OLT." They also noted that "RVEDVI was better correlated with PVi than with other static filling pressure[s], such as CVP or PAOP," therefore giving a "safer, faster, and better estimate of fluid responsiveness."
The researchers noted as a limitation that the quality of the signal from which PVi is calculated is "critically based on peripheral perfusion, which may be significantly affected by factors such as extreme low cardiac output, drug-induced vasoconstriction, and hypothermia." The researchers also noted that further studies are needed to investigate the application and effectiveness of additional dynamic hemodynamic parameters such as SVV and PPV during OLT.
References
1. Lee HC, Tsai YF, Tsai HI, Chung PC, Yu HP, Lee WC, Lin CC. Pulse Oximeter-Derived Pleth Variability Index is a Reliable Indicator of Cardiac Preload in Patients Undergoing Liver Transplantation. Transplant Proc. 2016 May;48(4):1055-8.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo PVi®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo PVi, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


New Study Assesses Utility of 1st Generation Masimo Pronto® Pulse CO-Oximeter® with SpHb® Spot Check Technology in Evaluating Pediatric Trauma Patients
Irvine, California – July 14, 2016 – Masimo (NASDAQ: MASI) announced today that in a new study using the 1st Generation Masimo Pronto® Pulse CO-Oximeter®, which noninvasively measures total hemoglobin (SpHb®), researchers found that noninvasive and invasive measurements correlated well and that "given the rapid availability of results and the lack of requirement of venipuncture, noninvasive hemoglobin monitoring may be a valuable adjunct in the initial evaluation and monitoring of pediatric trauma patients."1
Pronto features rainbow SET™ technology, allowing for the noninvasive spot checking of SpHb, oxygen saturation (SpO2), pulse rate (PR), and perfusion index (Pi).
In the study, published online in the Journal of Trauma and Acute Care Surgery and conducted at the University of Tennessee Health Science Center, Le Bonheur Children's Hospital, Dr. Mark Ryan and colleagues evaluated noninvasive SpHb measurement accuracy relative to current invasive and point-of-care testing in pediatric trauma patients. They performed a prospective observational trial involving 114 patients under age 17, measuring hemoglobin levels with a point-of-care device (i-STAT) and a lab analyzer (Sysmex XN Series). Noninvasive hemoglobin measurement, using 1st Generation Pronto, was performed within 15 minutes of phlebotomy.
Of the 114 patients, SpHb was successfully measured 89% of the time. Mean lab hemoglobin was 12.6 ± 1.9 and mean SpHb was 12.3 ± 1.6 (mean point-of-care hemoglobin was 12.2 ± 2.0). Bland-Altman analysis showed the limits of agreement between lab hemoglobin and non-invasive SpHb to be -2.9 and 1.9, with a mean difference of -0.49.
The researchers concluded that they were able to demonstrate "low bias and strong correlation between hemoglobin measurements using a noninvasive monitor, a point-of-care testing device, and laboratory co-oximeter in pediatric trauma patients." They noted that noninvasive SpHb testing "may be most effective in determining when invasive testing of hemoglobin is warranted."
Key limitations to the study include: the majority of the hemoglobin values measured were within normal limits; limited data on injury severity, morbidity, and mortality to understand their effects on device accuracy and precision; data is based on a convenience sample depending on availability of certified personnel; and no assessment on the effects of pre-hospital administration of intravenous fluids.
The researchers noted that "further study is required to determine the clinical utility of the [Pronto] device during the initial assessment and its accuracy in evaluating hemoglobin levels in hemodynamically unstable patients." They stated that SpHb measurements should not be relied upon alone to determine active hemorrhage or the need for transfusion, and suggested the following reasons a noninvasive measurement could not be taken in 11% of the study's participants: severe anemia; patients who were normotensive but tachycardic; patients unreadable due to the presence of nail polish; and cold extremities or low signal IQ without hemodynamic instability or anemia.
The University of Tennessee Health Science Center received equipment from Masimo to support the data collection for this study.
References
1. Ryan, Maxwell, Manning, Jacobs, Bachier-Rodriguez, Felizm, and Williams. "Noninvasive hemoglobin measurement in pediatric trauma patients." Journal of Trauma and Acute Care Surgery. DOI: 10.1097/TA.0000000000001160. E-published ahead of print.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Pronto® Pulse CO-Oximeter with SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Pronto Pulse CO-Oximeter with SpHb, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.



One of the World's Leading Centers for Cardiovascular Medicine & Transplantation Adopts Masimo's SedLine® Brain Function Monitoring and O3™ Regional Oximetry
NEUCHATEL, Switzerland – July 7, 2016 – Masimo (NASDAQ: MASI) announced today that Deutsches Herzzentrum Berlin (DHZB / German Heart Center Berlin) in Berlin, Germany has adopted Masimo's SedLine® Brain Function Monitoring and O3™ Regional Oximetry technologies to monitor patients in the operating room and intensive care unit.
"Our non-profit hospital performs over 32,000 treatments each year. Easy access to advanced parameters and the data they provide are very important for our patients, many of whom are undergoing especially complex and critical operations," stated Prof. Dr. Hermann Kuppe, Director of the Department of Anesthesiology at DHZB. "Masimo's technology allows us to access multiple parameters quickly and noninvasively from one monitor."
SedLine brain function monitoring provides four channels of electroencephalogram (EEG) to help clinicians better understand the effects of anesthesia on the brain. SedLine monitors the effects of anesthesia and sedation by monitoring electrical activity on both sides of the brain. O3 regional oximetry provides regional oxygen saturation (rSO2) and reference arterial oxygen saturation (SpO2) information, helping clinicians detect regional hypoxemia that pulse oximetry alone can miss.
"The innovative treatment programs at Deutsches Herzzentrum Berlin have helped it to become recognized as one of the world's leading centers for cardiovascular medicine and transplantation. They have doctors, medical students, and scientific researchers from around the world apply to visit the hospital as clinical observers every year," stated Joe Kiani, Founder and CEO of Masimo. "We are happy to see them adopt our SedLine and O3 technologies and are impressed by their focus on patient safety."
DHZB is a specialist hospital dedicated to the diagnosis and treatment of cardiovascular and thoracic diseases, the implantation of mechanical circulatory support systems, and heart and lung transplantations. A cooperative partnership with Charite-Universitatsmedizin Berlin, each year the hospital performs more than 6,700 operations, including 2,400 operations using heart-lung machines. DHZB treats more than 7,200 inpatients and 24,000 outpatients annually. The hospital has 194 beds, 71 of which are in intensive care. Since 2002, DHZB has received certification by KTQ, the national quality control board, recognizing the high standard of care DHZB provides. DHZB and its subsidiaries employ 1,300 people, including 190 doctors and almost 500 nurses.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7® wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine® and O3™ technologies. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine® and O3™ technologies, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Deutsches Herzzentrum Berlin
Christian Maier
Phone: + 49 03 04 5931211
Email: cmaier@dhzb.de
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Announces FDA 510(k) Clearance for Radius-7® with rainbow® Technology, Including Continuous SpHb®
Radius-7 is the First Wearable, Tetherless, Noninvasive rainbow® Monitor
IRVINE, California – June 28, 2016 – Masimo (NASDAQ: MASI) announced today FDA 510(k) clearance for Radius-7® – the first and only wearable, tetherless, noninvasive rainbow® monitor. Radius-7, which connects to the Root® patient monitoring and connectivity platform, is now available in the U.S. with breakthrough Masimo rainbow® technology. With this clearance, Radius-7 with Root now enables noninvasive monitoring of more than 10 parameters, including, for the first time in a wearable device, total hemoglobin (SpHb®), a breakthrough measurement that noninvasively and continuously measures hemoglobin concentration.
-

Masimo Root® with Radius-7® with rainbow®
SpHb monitoring may provide additional insight to the directional trend of hemoglobin between invasive blood samplings – when the SpHb trend is stable and the clinician may otherwise think hemoglobin is decreasing; when SpHb trend is rising and the clinician may otherwise think hemoglobin is not rising fast enough; or when the SpHb trend is decreasing and the clinician may otherwise think hemoglobin is stable. SpHb may thus help clinicians make more timely and informed decisions, and has been shown to help clinicians provide more timely blood transfusions* and reduce blood transfusions in cases such as neurosurgery and orthopedic surgery.1,2
Professor Christer Svensen, Professor of Anesthesiology and Intensive Care at the Karolinska Institute in Stockholm, Sweden, who has been using Radius-7 as part of a research study, commented, "We are currently performing a noninvasive continuous study monitoring respiratory rate, heart rate and saturation for all patients admitted to a surgical ward. Additionally, we are monitoring SpHb for selected postsurgical patients, which can be extremely beneficial because it can provide insight into hemoglobin trends between invasive blood samplings. Such insight may lead clinicians to confirm trends by performing blood draws sooner than they might otherwise have done, which may then suggest the need to intervene."
For the first time, it is possible to offer patients freedom of movement while providing such important monitoring, and studies have shown that patient mobility is a key factor in more rapid patient recovery.3,4 When monitoring ambulating patients, Radius-7 communicates to Root at the bedside and thereby to Masimo Patient SafetyNet™ to alert clinicians of critical changes in oxygen saturation, pulse rate, respiration, and hemoglobin, among other parameters. Radius-7 is lightweight, weighing only 0.34 lbs, and attaches to the arm, thus allowing untethered monitoring whether a patient is in or out of bed – which also reduces the need for nursing assistance, as there is no need to disconnect from or reconnect to a bedside monitor. Each Radius-7 comes with two "hot-swappable" rechargeable battery modules (one with the patient, one charging), each with a battery life of 12 hours, minimizing monitoring interruption.
"Never before could patients be monitored for such key parameters as continuous SpHb, which can help clinicians make more timely and informed blood management decisions, while patients are fully mobile. Previous wearable patient monitors were hampered by a limited range of measurements and false alarms due to motion," said Joe Kiani, Founder and CEO of Masimo. "Root with Radius-7 with rainbow SET, coupled with Patient SafetyNet for mobile clinician notification, is now an even more versatile and powerful monitoring system, all while promoting freedom of patient movement and quicker recovery times."
*Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgement considering, among other factors: patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
References
1. Ehrenfeld JM et al. J Blood Disorders Transf. 2014. 5:9. 2.
2. Awada WN et al. J Clin Monit Comput. 2015 Feb 4.
3. Needham D, Korupolu R, Zanni J, Pradhan P, Colantuoni E, Palmer J, Brower R, Fan E. "Early Physical Medicine and Rehabilitation for Patients With Acute Respiratory Failure: A Quality Improvement Project." Archives of Physical Medicine and Rehabilitation. Vol 91, Issue 4, PP 536–542, April 2010.
4. Ronnenbaum J, Weir J, Hilsabeck T. Earlier mobilization decreases length of stay in the intensive care unit. J Acute Care Phys Ther. 2012;3(2):204-210.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo's Radius-7®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo's Radius-7®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.



New SunTech Medical CT40 Vital Signs Device to Feature Masimo SET® Pulse Oximetry Technology
NEUCHATEL, Switzerland and EYNSHAM, England – June 23, 2016 – Masimo (NASDAQ: MASI) and SunTech Medical, a Halma (LSE:HLMA) company, jointly announced today the integration of Masimo SET® pulse oximetry technology into SunTech's CT40, a next generation spot-check vital signs device.
Masimo Signal Extraction Technology (SET®) Measure-through Motion and Low Perfusion™ pulse oximetry measures oxygen saturation (SpO2), pulse rate, and perfusion index. Masimo SET® has been shown to significantly reduce false alarms and accurately monitor for true alarms1,2 and is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world.
The SunTech CT40 is an ideal, affordable solution for clinical-grade spot-check measurements of blood pressure, temperature and pulse oximetry in hospitals and clinics. Ambulatory care, long-term care and low-acuity hospital departments can easily implement this versatile, user-friendly vital signs device that offers both advanced features and digital connectivity. The modular design allows clinicians to easily make adaptations to the device while in the field, adding thermometry, SpO2 and Wi-Fi as needed. Other advanced features include the ability to transmit measurement data directly from the device, in accordance with HL7 messaging protocols, as well as BP Averaging Mode - an increasingly important component of accurate blood pressure measurement, as evidenced by the recently published SPRINT Study from the National Institutes of Health (NIH), which specified that a mean of three office BP measurements would be used to establish target BPs for Standard Group participants.3
"Both SunTech and Masimo share industry-leading technologies within our respective areas of expertise," said Kenny Andersen, VP of Strategic Product Development at SunTech Medical. "Partnering with them on our next-generation vital signs platform is a very natural strategy. Masimo SET pulse oximetry is the benchmark for clinical performance, just as SunTech Advantage technology is for blood pressure. We look forward to a long and successful partnership."
Rick Fishel, Masimo's President of Worldwide OEM Business and Strategic Development, stated, "SunTech Medical is a recognized global leader and worldwide supplier of non-invasive blood pressure measurement technology. Combining best-in-class measurement technologies that provide superior performance, accuracy, and affordability, in flexible and cost effective monitors, supports our organizations' mutual vision to offer solutions that help clinicians improve patient outcomes and reduce the cost of healthcare delivery. We are very pleased to partner with SunTech Medical and that Masimo SET pulse oximetry is the preferred pulse oximetry offering in the highly versatile and cost-effective SunTech CT40 monitor."
The SunTech CT 40 with Masimo SET® technology has a CE Mark. It is not FDA cleared and is not available for sale in the United States.
References
1. Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers". J Clin Anesth. 2012 Aug;24(5):385-91.
2. Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2.
3. The SPRINT Study Research Group. The design and rationale of a multi-center clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. October 2014; 11(5): 532-546.
About Masimo
SunTech Medical, a Halma company, has been the preeminent supplier of clinical-grade blood pressure monitoring products and technologies for nearly 30 years. More than 75 companies trust SunTech Medical's OEM non-invasive blood pressure solutions for their patient monitoring needs. SunTech Medical produces the leading cardiac stress test blood pressure monitor and is the world's foremost manufacturer of ambulatory blood pressure monitoring products. SunTech Medical also offers solutions for in-office blood pressure monitoring as well as a complete line of blood pressure cuffs designed for general and specific applications.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET® pulse oximetry. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET® pulse oximetry, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
SunTech Medical
Elliott Holloway
Phone: (919) 654-2366
Email: eholloway@suntechmed.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Announces FDA 510(k) Clearance for O3™ Regional Oximetry
Irvine, California - June 13, 2016 – Masimo (NASDAQ: MASI) announced today FDA 510(k) clearance for O3™ regional oximetry. Regional oximetry, also referred to as tissue or cerebral oximetry, may help clinicians monitor cerebral oxygenation in situations in which pulse oximetry alone may not be fully indicative of the oxygen in the brain due to various factors, such as the type of clinical procedure being performed.
-
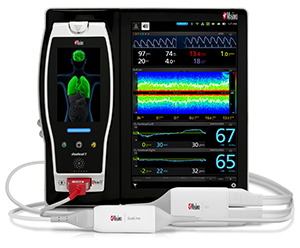
Masimo Root® with O3™ Regional Oximetry and SedLine® Brain Function Monitoring
O3 regional oximetry uses near-infrared spectroscopy (NIRS) to continuously monitor absolute and trended regional tissue oxygen saturation (rSO2) in the cerebral region. In a study on 27 subjects published in Anesthesia and Analgesia in 2014, Dr. Daniel Redford of the University of Arizona compared cerebral oxygen saturation measurements obtained from O3 with saturations obtained from blood samples (SavO2) through induced hypoxia.1 O3 regional oximetry provided absolute root-mean-squared error of 4% and relative root-mean-squared error of 2.1%.1 This study did not require that end tidal carbon dioxide (EtCO2) levels be fixed in the study protocol, allowing the rSO2 measurement to be responsive to changes in tissue oxygen saturation due to changes in CO2 in the blood. Follow up studies with O3 extended the subject pool to 74 subjects and demonstrated that O3 maintained its absolute and relative accuracy.2
"O3 regional oximetry delivers again on Masimo's technical prowess and gives clinicians access to valuable, accurate data about cerebral oxygen saturation," stated Joe Kiani, Founder and CEO of Masimo. "With the addition of O3 regional oximetry to the Root platform, clinicians can simultaneously access rSO2 and other measurements including SedLine brain function monitoring, Masimo SET SpO2, PVi, and SpHb monitoring – all in one monitoring platform."
O3 regional oximetry is currently intended for use with adults weighing 40 kg (88 lbs) or greater.
References
1. Redford D, Paidy S, Kashif F. Absolute and Trend Accuracy of a New Regional Oximeter in Healthy Volunteers During Controlled Hypoxia. Anesth Analg. 2014 Dec;119(6):1315-1319.
2. Masimo FDA submission data on file.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo O3™ Regional Oximetry. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo O3™ Regional Oximetry, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Announces Full Market Release of Next Generation SpHb® Spot Check Pronto®
NEUCHATEL, Switzerland – June 8, 2016 – Masimo (NASDAQ: MASI) announced today the full market release outside the U.S. of the Pronto® Pulse CO-Oximeter® with Next Generation SpHb® Spot Check technology. Next Generation Pronto features rainbow SET™ technology, for noninvasive spot checking of total hemoglobin (SpHb), oxygen saturation (SpO2), pulse rate (PR), and perfusion index (Pi).
-

Masimo Pronto® Pulse CO-Oximeter® with Next Generation SpHb® and rainbow® DCI®-mini Reusable Sensor
In addition to the Next Generation SpHb technology, Masimo has also released the rainbow® DCI®-mini reusable sensor to accompany the Pronto. The DCI-mini is a universal sensor usable on patients greater than 3 kg, making Pronto an even more versatile solution.
The Next Generation SpHb technology in Pronto offers motion tolerance and a 40% reduction in time to display SpHb results. Field accuracy has been improved in the range of 6 to 11 g/dL, and is comparable to certain portable invasive point of care devices.
"This is a significant enhancement to the noninvasive measurement we introduced 7 years ago," stated Joe Kiani, Founder and CEO of Masimo. "Since then, all of the clinical outcome studies we're aware of on our continuous SpHb technology have been positive,1-3 which is something that couldn't be said for standard pulse oximetry before the introduction of Masimo SET SpO2. We will continue to improve SpHb until it has the same measure-through motion and low perfusion performance as our SET SpO2 technology."
An upgrade program will be available for qualified existing Pronto customers. Pronto with Next Generation SpHb and the DCI-mini reusable sensor have not received FDA 510(k) clearance and are not currently available for sale in the United States.
References
1. Ehrenfeld JM, Henneman JP, Bulka CM, Sandberg WS. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial .2014. J Blood Disorders Transf. 5:237.
2. Awada WN, Mohmoued MF, Radwan TM, Hussien GZ, Elkady HW. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Dec;29(6):733-40.
3. Ponsonnard S, Yonnet S, Marin B, Cros J, Ben Miled S, Nathan N. "Continuous Hb and plethysmography variability index (PVi) monitoring is associated to a decreased mortality at the scale of a whole hospital." Proceedings of the European Society of Anaesthesiology's Euroanaesthesia 2015 Annual Congress, May 30-June 2, Berlin, Germany, 16AP3-2, Room A1 – Poster Abstract Presentation Session, e-Board 8.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect (MOC-9) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at http://www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo's Pronto® Pulse CO-Oximeter®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo's Pronto Pulse CO-Oximeter, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.



One of India's Premier Maternal Care Specialists, Motherhood Hospitals, Standardizes on Masimo SET® Technology
Bangalore, India and Irvine, California - June 2, 2016 - Masimo (NASDAQ: MASI) announced today that Motherhood Hospitals, a maternal care specialist with five hospitals in three cities in India, has standardized on Masimo SET® (Signal Extraction Technology®) pulse oximetry at all locations. Motherhood Hospitals will also be screening for Critical Congenital Heart Disease (CCHD) using Masimo's technology.
"We evaluated several technologies and found Masimo SET pulse oximetry to be the most reliable even in the most challenging conditions," said Dr. Mohammed Rehan Sayeed, Chairman, Motherhood Hospitals. "This entire standardization exercise was driven by the clinical staff."
Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry measures oxygen saturation (SpO2), pulse rate (PR), and perfusion index (Pi), and helps clinicians monitor more than an estimated 100 million patients a year.1 In a study of newborns, researchers found CCHD was identified by physical examination alone 63% of the time, and when Masimo SET® pulse oximetry was added to the physical exam screening, researchers found that clinicians were able to identify CCHD 83% of the time - a 31% increase.2
"We are happy to see the team at Motherhood Hospitals, which conducts research into how to improve the health and well-being of neonates in India, select our SET technology," said Joe Kiani, Founder and CEO of Masimo. "We hope to work with Motherhood Hospitals to standardize universal screening for CCHD in newborns. We are seeing more and more hospitals in this region of the world realize the important role reliable pulse oximetry plays in patient care and safety."
Motherhood Hospitals, a premium birthing boutique, provides a variety of services to expecting women and newborns in India, specializing in obstetrics, gynecology, neonatology, pediatrics, fetal medicine, fertility, and cosmetology. At their combined locations in Bangalore, Chennai, and Hyderabad, they perform over 6,000 deliveries annually.
References
1. Masimo data on file.
2. de-Wahl Granelli A, Wennergren M, Sandberg K, Mellander M, Bejlum C, Inganäs L, Eriksson M, Segerdahl N, Agren A, Ekman-Joelsson BM, Sunnegardh J, Verdicchio M, Ostman-Smith I. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009 Jan 8;338:a3037.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect (MOC-9) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo to Showcase Next Generation SedLine® Brain Function Monitor at 2016 Euroanaesthesia Congress
SedLine, Which Helps Clinicians Improve Anesthetic Management, Receives CE Mark
Neuchatel, Switzerland – May 26, 2016 – Masimo (NASDAQ: MASI) announced today the CE mark and scheduled full market release of Next Generation SedLine® Brain Function Monitoring technology at the European Society of Anaesthesiology's (ESA) 2016 Euroanaesthesia Congress in London, England.
-
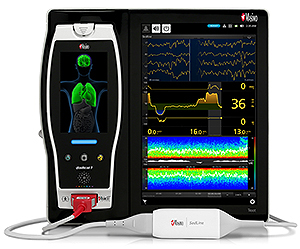
Masimo Root® with Next Generation SedLine®
Next Generation SedLine enhances how Masimo's processed EEG parameter, the Patient State Index (PSi), responds in challenging situations, addressing many of the concerns raised with quantitative EEG while bolstering brain function monitoring's support of anesthetic management.
Next Generation PSi uses Masimo's breakthrough Parallel Engines and Adaptive Signal Processing technology and provides the following enhancements:
- Less susceptibility to electromyography (EMG) interference by extracting a clearer EEG signal even in the presence of EMG
- Improved PSi performance in low power EEG cases
These improvements build upon the existing benefits of SedLine technology:
- Four simultaneous EEG leads to enable continuous assessment of both sides of the brain
- Density Spectral Array (DSA) offers easy-to-interpret, high-resolution display of bi-hemispheric activity
- Multiple screen views expand information while enabling customization in the OR and ICU
- Electrocautery resistance
"We are delighted to unveil Next Generation SedLine," said Joe Kiani, Founder and CEO of Masimo. "Next Generation PSi takes advantage of Masimo's signal processing prowess and promises to do for brain function monitoring what SET did for pulse oximetry."
Next Generation SedLine has not received FDA 510(k) clearance and is not currently available for sale in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect (MOC-9) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.



Two Fire Departments Honored for Excellence in Emergency Medical Services
Fire-Service Based EMS Awards Presented at 28th Annual National Fire and Emergency Services Dinner
Washington, DC and Irvine, CA – May 19, 2016 – The Congressional Fire Services Institute (CFSI) and Masimo (NASDAQ: MASI) honored two fire departments for best practices and innovative solutions in the delivery of emergency medical services with the Excellence in Fire Service-Based EMS Award. The award presentation took place on May 5th in Washington, DC. Masimo is proud to be a co-sponsor of this award.
- The Olathe Fire Department in Olathe, Kansas, established the Mobile Integrated Health (MIH) team to provide better treatment in the field for patients. With the support of local government, community partners, and the Olathe Medical Center, the MIH team reduced the number of patient transports to emergency rooms, monitored the progress of these patients, and scheduled follow-up visits for evaluation and continued treatment.
- Kittitas Valley Fire and Rescue (KVFR) in Kittitas, Washington, implemented new programs that are reducing demand for emergency services while providing better care and treatment for its citizens. KVFR has launched a community paramedicine program to provide alternative medical care options to alleviate pressure on the 911 system and improve patient outcomes. They conduct educational programs for students, law enforcement, and other community members that address the dangers of alcohol and drug abuse. In addition, KVFR has developed a new fee structure and an educational program to address a rising call volume for non-injury lift assistance requests at assisted living facilities.
"CFSI takes great pride in co-sponsoring the Excellence in Fire Service-Based EMS Awards with Masimo," said Dr. William F. Jenaway, Ph.D., President of CFSI. "Through this awards program, we are able to recognize innovations in the delivery of emergency medical services throughout the nation. The recipients of the 2016 award – Kittitas Valley (WA) Fire and Rescue Department and the Olathe (KS) Fire Department – certainly demonstrated themselves worthy of this recognition by developing new programs to enhance their delivery of emergency medical services. Congratulations to both departments."
"Firefighters and emergency services personnel are our heroes. They put their lives on the line for us every day and we want to make sure they have the technology and community support they need to keep them safe and help them better care for the citizens they protect," said Joe Kiani, Founder and CEO of Masimo. "Masimo is honored to co-sponsor this awards program with CFSI and to recognize these two departments for their innovative achievements."
Approximately 1,600 fire and emergency services leaders attended the 28th Annual National Fire and Emergency Services Dinner to pay tribute to the dedication and commitment of the nation's fire and emergency services providers. Hosted by CFSI, the annual dinner benefits the mission of the nonprofit policy organization, which is designed to educate members of Congress about fire and life safety issues. This year's guest speaker was the Honorable Tom Vilsack, U.S. Secretary of Agriculture.
The Excellence in Fire Service-Based EMS Awards Program was established in 2010 to recognize volunteer, career, and combination fire departments for excellence in and enhancements to the delivery of emergency medical services. By showcasing their best practices, the awards program provides ideas for other fire departments to consider implementing as they seek to improve their fire service-based EMS systems.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect (MOC-9) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
CFSI
Bill Webb, Executive Director
Phone: (202) 371-1277
Email: bwebb@cfsi.org
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Reports First Quarter 2016 Financial Results
Q1 2016 Highlights (compared to Q1 2015):
- Total revenue, including royalties, rose 10.8% to $171.2 million
- Product revenue rose 10.8% to $163.3 million
- Masimo rainbow® revenue rose 39.2% to $16.9 million
- SET® and rainbow® SET® units shipments were 46,300
- GAAP net income of $27.6 million, or $0.53 per diluted share versus $20.5 million, or $0.38 per diluted share in the year-ago period
Irvine, California, May 4, 2016 – Masimo (NASDAQ: MASI) today announced its financial results for the first quarter ended April 2, 2016.
First quarter 2016 product revenues rose 10.8% to $163.3 million, compared to $147.4 million for the first quarter of fiscal year 2015, and total revenue, including royalties, rose 10.8% to $171.2 million, up from $154.5 million for the first quarter of fiscal year 2015.
The company's worldwide direct product revenue in the first quarter of 2016 rose by 12.7% compared to the same period in 2015 and represented 86.3% of product revenue. OEM sales, which accounted for 13.7% of product revenue, rose by 0.2% to $22.3 million in the first quarter of 2016 compared to the same period in 2015. Revenue from sales of Masimo rainbow® products rose by 39.2% to $16.9 million in the first quarter of 2016, compared to the same period in 2015.
GAAP net income for the first quarter of 2016 was $27.6 million, or $0.53 per diluted share, compared to GAAP net income of $20.5 million, or $0.38 per diluted share, in the first quarter of 2015. During the first quarter of 2016, the company shipped 46,300 SET® pulse oximeters and rainbow® pulse CO-Oximeters, excluding handheld units. Masimo estimates its worldwide installed base as of April 2, 2016 to be 1,438,000 units, up 7.3% from 1,340,000 units as of April 4, 2015.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "We are happy to report results for the first quarter exceeded our expectations. Our strong revenue growth is the result of increasing demand for both our SET® Pulse Oximetry and rainbow SET™ Pulse CO-Oximetry™ technologies, as well as increasing demand from our new products. Given the strong Q1 results and our continued optimism about the rest of the year, we are happy to be in a position to raise our full year 2016 financial guidance."
During the first quarter of 2016, the company generated $18.8 million in cash from operations and as of April 2, 2016, total cash and cash investments were $139.9 million compared to $132.3 million as of January 2, 2016. Also, during the first quarter of 2016, the company repurchased approximately 1.1 million shares of stock for $42.9 million.
2016 Financial Guidance
Masimo today is updating its 2016 financial guidance. Masimo now expects fiscal 2016 total revenues to be approximately $677 million, up from $670 million. Total fiscal 2016 product revenues are now expected to be approximately $647 million, up from $640 million, while royalty revenue expectations have not changed from the previous estimate of approximately $30 million. Masimo now also expects its fiscal 2016 GAAP earnings per diluted share to be $1.83, up from $1.69. Masimo will provide additional financial information during the conference call today. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be available online from the investor relations page of the company's website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 95558540. After the live webcast, the call will be available on Masimo's website through May 18, 2016. In addition, a telephonic replay of the call will be available through June 3, 2016. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 95558540.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVI®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect™ (MOC-9™) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year 2016 total, product and royalty revenues and GAAP earnings per diluted share; demand for our products; anticipated revenue and earnings growth; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow® SET® products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
| Investor Contact: Eli Kammerman Phone: (949) 297-7077 Email: ekammerman@masimo.com | Media Contact: Irene Paigah Phone: (858) 859-7001 Email: irenep@masimo.com |
|---|
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation
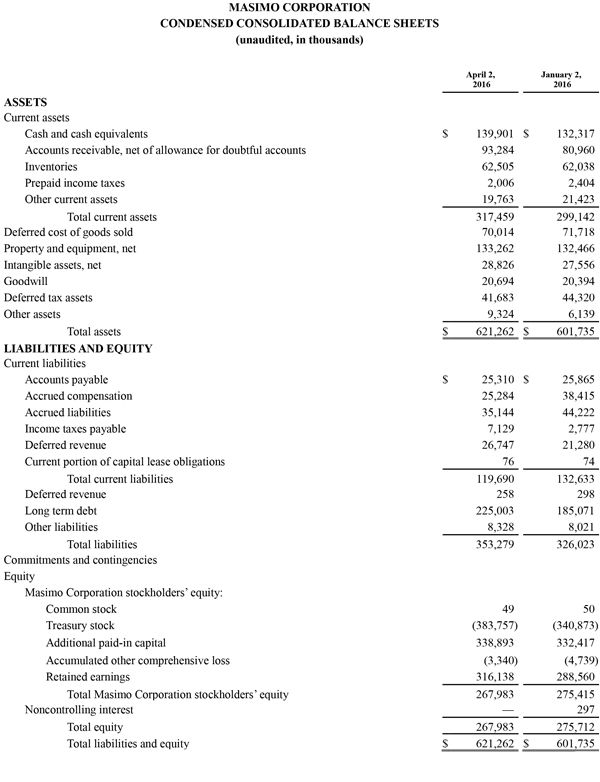
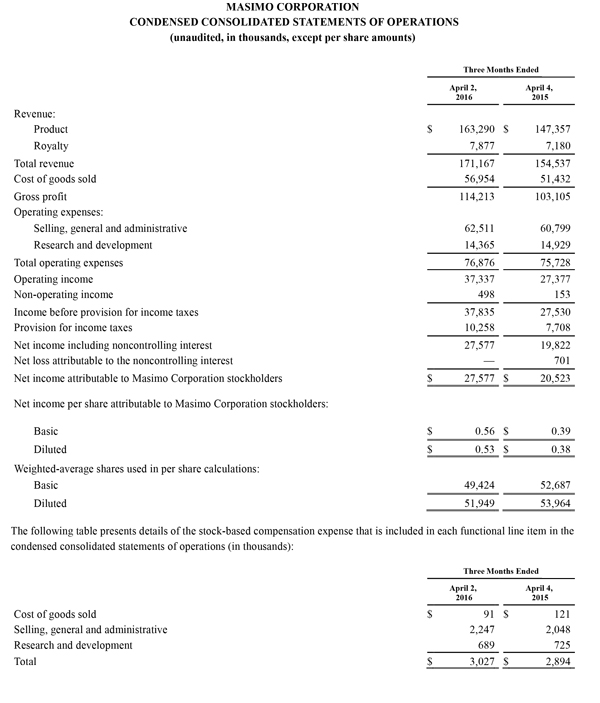
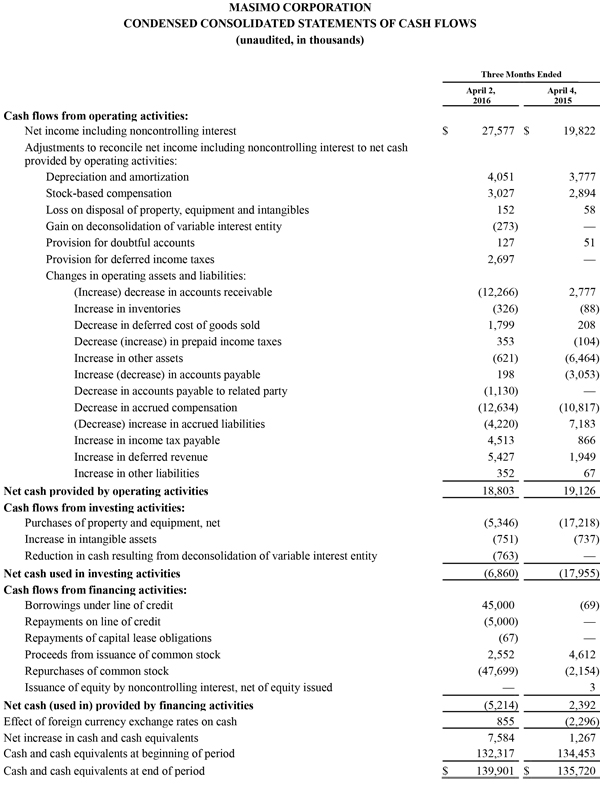


One of France's University Hospitals Adopts Masimo Radical-7®, Including SpHb®, PVi®, and SET® Pulse Oximetry
Neuchatel, Switzerland – April 21, 2016 – Masimo (NASDAQ: MASI) announced today that Centre Hospitalier Universitaire (CHU) de Limoges, in Limoges, France has installed Masimo's Radical-7®, including noninvasive, continuous hemoglobin (SpHb®), Pleth Variability Index (PVi®), and SET® pulse oximetry technology, to monitor surgical patients and critical patients in the post-anesthesia care unit and intensive care unit.
"For three years, we tested SpHb and PVi on every surgical patient in our facility and then compared this data to the previous period without this technology," said Professor Nathalie Nathan, Head of the Department of Anesthesiology at CHU Limoges. "In this retrospective review of before and after periods across the entire hospital, in different clinical settings and with many different practitioners, we found that there was a notable reduction in mortality in surgeries lasting longer than 2 hours and patients in the 60+ age range."
SpHb is a breakthrough measurement that noninvasively and continuously measures total hemoglobin in the blood, and offers real-time visibility into changes – or lack of changes – in hemoglobin between invasive blood samples. PVi is a measure of dynamic changes in Perfusion Index (Pi) that occur during one or more complete respiratory cycles, and may show changes that reflect physiologic factors such as vascular tone, circulating blood volume, and intrathoracic pressure excursions. PVi is noninvasive, easily obtained and has no incremental procedural cost.
"According to the World Health Organization, France ranks number one in the world in patient safety. For over 40 years, CHU Limoges has been recognized as a leading hospital in France for patient care, training, research and innovation," stated Joe Kiani, Founder and CEO of Masimo. "We are impressed with the format of this three-year evaluation and happy to see Limoges incorporate SpHb and PVi in the care of their patients."
CHU Limoges is a university hospital that includes four locations and one nursing home. Dupuytren (866 beds) hosts an emergency department, Jean Rebeyrol (459 beds) handles rehabilitation patients, Cluzeau (59 beds) specializes in respiratory diseases, diabetes, endocrinology and metabolic diseases, and Mother & Child (197 beds) specializes in pediatric and gynecological emergencies.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2", pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect (MOC-9) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo's SpHb®, PVi®, and SET® technologies. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo's SpHb®, PVi®, and SET® technologies, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo to Report First Quarter 2016 Financial Results after Market Close on Wednesday, May 4
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., April 20, 2016 – Masimo (NASDAQ: MASI) announced today that it will release financial results for the first quarter ended April 2, 2016, after the market closes on Wednesday, May 4, 2016. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 95558540. After the live webcast, the call will be available on Masimo's website through May 18, 2016. In addition, a telephonic replay of the call will be available through June 3, 2016. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 95558540.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect (MOC-9) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Eli Kammerman
Masimo
Phone: (949) 297-7077
Email: ekammerman@masimo.com
Media Contact:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


New Clinical Study Reports Relationship Between Masimo's Oxygen Reserve Index (ORI™) and Arterial Partial Pressure of Oxygen During Surgery
Neuchatel, Switzerland – March 31, 2016 – Masimo (NASDAQ: MASI) announced today that a new retrospective study reports the relationship between Masimo's Oxygen Reserve Index (ORI™) and arterial partial pressure of oxygen (PaO2) during surgery. The study suggests that ORI may provide an advanced indication of impending desaturation in adults undergoing surgery based on trends in the relationship between ORI and PaO2.1
-
Masimo Root® with ORI™
ORI, Masimo's 11th rainbow® parameter2, which is available outside of the United States, is a relative index of the PaO2 operating in the range of 100 to 200 mm Hg. ORI can provide a calculated oxygen reserve index that may add to information from pulse oximetry when oxygen saturation (SpO2) is >98%. ORI is intended to supplement, not replace, SpO2 monitoring and PaO2 measurements.
In the study, published in the journal Anesthesia & Analgesia and conducted at Loma Linda University, Dr. Applegate and colleagues studied 106 patients in which arterial catheterization and intraoperative arterial blood gas analysis were planned during surgery. Multiwavelength Pulse CO-oximetry data from multiple sensors on each patient were continuously collected (Masimo Radical-7® with Root®). A retrospective analysis was then conducted to evaluate the ORI to PaO2 relationship.
Regression analysis showed a positive ORI to PaO2 relationship for PaO2 values up to 240 mm Hg (r2 = 0.536), but not for PaO2 over 240 mm Hg (r2 = 0.0016). Measured PaO2 was ≥100 mm Hg for all ORI values over 0.24, and when ORI was higher than 0.55, the measured PaO2 was ≥150 mm Hg in 96.6% of samples.
The investigators concluded, "This study suggests that intraoperative ORI can distinguish PaO2 between 100 and 150 mm Hg when SpO2 is >98%. Decreases in ORI to near 0.24 may provide advance indication of falling PaO2 when SpO2 is still >98% and above the PaO2 level at which SaO2 [arterial oxygen saturation] declines rapidly. Usefulness of ORI >0.24 to distinguish PaO2 ≥100 mm Hg and ORI >0.55 to distinguish PaO2 ≥150 mm Hg should be tested prospectively in a range of clinical settings." Additionally, the investigators noted that "the clinical utility of interventions based on continuous ORI monitoring should also be studied prospectively," and that other clinical or feasibility limitations may not have been identified. The authors also noted that the study was limited by a small number of subjects and samples with PaO2 values <100 mm Hg, and that the study results did not apply to intensive care settings.
The Loma Linda University School of Medicine, Department of Anesthesiology received a grant from Masimo to support the data collection for this study.
Radical-7 with Root has a CE Mark with the ORI parameter. ORI has not received FDA 510(k) clearance and is not available for sale in the United States.
References
1. Applegate RL 2nd, Dorotta IL, Wells B, Juma D, Applegate PM. The Relationship Between Oxygen Reserve Index and Arterial Partial Pressure of Oxygen During Surgery. Anesth Analg. 2016 Mar 22. [Epub ahead of print] PubMed PMID: 27007078.
2. The 11 rainbow® parameters include: 1) oxygen saturation (SpO2); 2) Pulse rate; 3) Perfusion index (Pi); 4) Pleth Variability Index (PVi®); 5) Respiration Rate from the pleth (RRp®); 6) Total hemoglobin (SpHb®); 7) Oxygen Content (SpOC™); 8) Carboxyhemoglobin (SpCO®); 9) Methemoglobin (SpMet®); 10) Fractional oxygen saturation (SpfO2®); 11) Oxygen Reserve Index (ORI™).
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform with the Masimo Open Connect (MOC-9) interface. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo's Oxygen Reserve Index (ORI™). These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo's Oxygen Reserve Index (ORI™), contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


New Study Reports on Accuracy of Masimo's Blue® Sensors at Saturations Below 85% in Children with Cyanotic Congenital Heart Disease
Irvine, California – March 17, 2016 – Masimo (NASDAQ: MASI) announced today that a new study reported on the accuracy of Masimo's Blue® sensors as compared to Nellcor and Masimo Standard sensors for cyanotic children with blood oxygen saturation less than 85%.1 Masimo's Blue sensor was designed specifically for cyanotic infants and children with congenital heart disease.
The study was performed at the Lucile Packard Children's Hospital at Stanford University with the aim of determining, in children with cyanotic congenital heart disease, which of three different pulse oximeter sensors is the most accurate at lower blood oxygen saturation levels, when compared to the "gold standard" of arterial blood gas analysis with CO-oximetry. The three sensors were Masimo SET® with LNCS® sensor (Masimo Standard), Masimo SET® with Blue sensor (Masimo Blue), and Nellcor™ N-600 with MAX-I sensor (Nellcor).
In the study, published in the journal Pediatric Critical Care Medicine, Dr. Harris and colleagues collected data from 50 infants and children weighing 3–20 kg with baseline saturations under 90% (measured by pulse oximetry at the time of clinical assessment) undergoing surgical or catheterization procedures. Following standard care monitoring, which included placement of the Masimo Standard sensor, the Masimo Blue and Nellcor MAX-I sensors were placed on a thumb or great toe, or alternatively on a finger. Up to four arterial blood samples were taken from each subject.
Compared to the CO-oximeter saturations (SaO2) over all measurements, the mean error ± standard deviation of the saturation values from pulse oximetry (SpO2) for the Masimo Blue, Nellcor, and Masimo Standard sensors were 1.7% ± 3.3%, 1.7% ± 5.4%, and 2.4% ± 4.9%, respectively.
The investigators concluded that "the Masimo Blue sensor has improved accuracy at saturations 75–85% versus the Nellcor and Masimo Standard sensors," although the authors noted inaccuracies with all three sensors in this saturation range. The authors suggested there is a continued need for device manufacturers to calibrate and develop algorithms for pulse oximeters to have improved overall accuracy at saturations less than 85%. The authors further stated "clinical decisions at saturations below 85% should not be made by pulse oximetry alone; instead, co-oximetry and/or an arterial blood gas is warranted."
Study limitations included small sample size, difficulty collecting steady-state samples from the operating room and catheterization lab, and placement of the Masimo Standard sensor in nonstandard locations.
The institution where this study was performed received a grant from Masimo to partially support the data collection for this study.
References
1. Harris BU, Char DS, Feinstein JA, Verma A, Shiboski SC, Ramamoorthy C. Accuracy of Pulse Oximeters Intended for Hypoxemic Pediatric Patients. Pediatr Crit Care Med. 2016 Feb 24. [Epub ahead of print] PubMed PMID: 26914626.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive open architecture patient monitoring and connectivity platform designed to speed the pace of innovation and reduce the cost of care. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com. All published clinical studies on Masimo products can be found at www.masimo.com/cpub/clinical-evidence.htm.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo's Blue® sensor. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo's Blue® sensor, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Introduces SafetyNet™ Surveillance at HIMSS16
Patient Video Surveillance System Provides High-Definition Real-Time Imaging at the Bedside
Irvine, California – March 2, 2016 – Masimo (NASDAQ: MASI) today announced the debut of SafetyNet™ Surveillance at the HIMSS (Healthcare Information and Management Systems Society) 2016 Annual Conference and Exhibition in Las Vegas.
-
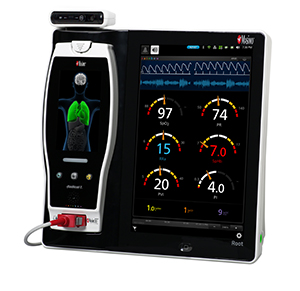
Masimo Root® with SafetyNet™ Surveillance
SafetyNet Surveillance is a video surveillance system that provides real-time video images of a patient's room, including the patient with connected monitoring devices, to a central station. Masimo SafetyNet Surveillance is a software option for Masimo's Patient SafetyNet system, adding existing communication technology to central monitoring.
The camera delivers a high-resolution, high-frame rate video feed to the Patient SafetyNet view-station. An external mechanical lens cap lets patients or their visitors disable the camera at any time. Two-way audio is available to allow the caregiver to listen and communicate with the patient. The system utilizes the existing hospital IT network and can provide viewing of images in the same care area.
"Video surveillance that is not cost prohibitive adds an exciting new dimension to hospital healthcare systems," said Joe Kiani, Founder and CEO of Masimo. "SafetyNet Surveillance combines innovations we take for granted in our everyday lives—real-time video and two-way audio communication—with top-tier noninvasive medical technology to deliver a holistic system cost effectively. We hope this enhancement to Patient SafetyNet will improve the quality and safety of patient care and help reduce costs."
For more information on Masimo and its products, go to http://www.masimo.com.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive open architecture patient monitoring and connectivity platform designed to speed the pace of innovation and reduce the cost of care. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo's SafetyNet™ Surveillance system. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including SafetyNet™ Surveillance, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Announces Iris™ Gateway at HIMSS16
Integrated Medical Device Data Management Solution Designed to Fill the Gaps in Electronic Medical Records
Irvine, California – March 1, 2016 – Masimo (NASDAQ: MASI) today announced the debut of Iris™ Gateway, a server-based software solution for integrating medical device data, at the HIMSS (Healthcare Information and Management Systems Society) 2016 Annual Conference and Exhibition in Las Vegas.
Data generated from medical devices remain captive within each device, and may not be captured in the patients' electronic medical records (EMR). Iris Gateway can provide a timely and cost-effective solution by connecting to existing medical devices and performing the required translations to move the data from the devices into EMRs. Iris Gateway can be deployed on a remote server farm or as a server appliance in the hospital.
The Iris ports on the Root® patient monitor allow other medical devices (such as infusion pumps, ventilators, patient monitors, and "smart" beds) to connect to Iris Gateway via Root. Data from these connected devices can be associated with the patient and entered into the patient's EMR, via Iris Gateway. Root with noninvasive blood pressure and temperature measurements, deployed at the bedside or on a roll stand, can chart these parameters at the point of care while also copying the data into an EMR.
"Iris Gateway is an important part of Masimo's goal of bridging medical device data silos," said Joe Kiani, Founder and CEO of Masimo. "It can be used to connect isolated devices to hospital EMR systems in high-acuity settings like the OR and the ICU or in low-acuity settings like medical-surgical units. When data from different medical devices is more readily available in the patient's electronic medical record, they may provide a clearer and more complete picture of the patient. At Masimo, we are dedicated to providing clinicians with vital information when they need it most."
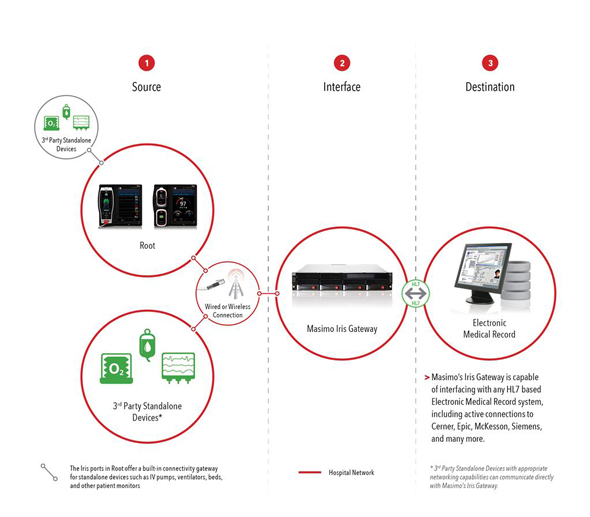
For more information on Masimo and its products, go to http://www.masimo.com.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive open architecture patient monitoring and connectivity platform designed to speed the pace of innovation and reduce the cost of care. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo's Iris™ Gateway. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Iris™ Gateway, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.



Manipal Hospitals is the First in India to Adopt Masimo Patient SafetyNet™
Irvine, California & Bengaluru, India – February 25, 2016 Masimo (NASDAQ: MASI) announced today that Manipal Hospitals is the first hospital in India to install Masimo Patient SafetyNet™, a remote monitoring and clinician notification system.
"With Patient SafetyNet, we are now able to monitor a certain set of patients 24x7," said Gopal Devanahalli, Chief Operating Officer of Manipal Hospitals. "One of the key features of this system is its proprietary Signal Extraction Technology (SET®) that helps significantly reduce false alarms."
"This is the technological advancement that we were awaiting," said Dr. Nagendra Swamy SC, MD, Senior President, Manipal Health Enterprises, and Group Medical Director & Chairman, Quality Council, Manipal Health Enterprises. "The technology will notify the hospital team when there are signs of deterioration and allow our patients to be monitored even though the nurse is not physically in the room with them."
Masimo Patient SafetyNet works in conjunction with Masimo bedside monitors which provide continuous and noninvasive monitoring of oxygen saturation (SpO2), pulse rate, respiration rate, and other parameters. When changes occur in the measured values, which may indicate deterioration in the patient's condition, in addition to bedside alarms, Masimo Patient SafetyNet automatically sends wireless alerts directly to clinicians. Continuous patient surveillance with Masimo Patient SafetyNet has been shown to reduce the need for rescue events and intensive care unit transfers in hospitals and, as a result, can reduce costs related to these events.1,2
"Manipal Hospitals treats close to 2 million patients annually and needed a monitoring system that they could trust would work in challenging patient populations," said Joe Kiani, Founder and CEO of Masimo. "We are excited to see this shift internationally with more and more hospitals worldwide recognizing the importance of integrating remote monitoring systems as a part of their patient safety initiatives."
For more information on Patient SafetyNet, go to http://www.masimo.com.
References
1. Taenzer A.H., Pyke J.B., McGrath S.P., Blike G.T. Anesthesiology. 2010 Feb;112(2):282-7.
2. Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring - The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online.
About Manipal Hospitals
As a pioneer in healthcare, Manipal Hospitals is among the largest hospital networks in India, serving over 2 million patients annually. Its focus is to develop an affordable tertiary care multispecialty healthcare framework through its entire multispecialty delivery spectrum and further extend it to homecare. With its flagship quaternary care facility located in Bengaluru, India, 8 tertiary care, 7 secondary care and 2 primary care clinics spread across India and abroad, today Manipal Hospitals successfully operates and manages 5,200+ beds. Manipal Hospitals provides comprehensive curative and preventive care for a multitude of patients from across the globe. Manipal Hospitals is the first in India to be awarded accreditation by the AAHRPP for ethical standards in clinical research activities. It is also NABL, NABH and ISO certified. Manipal Hospitals is also the most respected hospital company in India and the most patient recommended hospital in India by consumer survey.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive open architecture patient monitoring and connectivity platform designed to speed the pace of innovation and reduce the cost of care. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results or studies; risks related to our belief that Masimo's unique noninvasive measurement technologies and monitoring system solutions, including Masimo Patient SafetyNet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation, including the dental setting; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Anjana Chandran
Manipal Hospitals
Phone: +91-988-627-8400
Email: anjana.chandran@manipalhospitals.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo Announces FDA 510(k) Clearance for Root® with Noninvasive Blood Pressure and Temperature
Irvine, California – February 17, 2016 – Masimo (NASDAQ: MASI) announced today FDA 510(k) clearance for noninvasive blood pressure and temperature measurements for the Root® patient monitoring and connectivity platform.
Root with noninvasive blood pressure from SunTech Medical enables clinicians to measure arterial blood pressure for adult, pediatric, and neonatal patients, with three distinct measurement modes: spot-check, automatic interval, and stat interval. The temperature module from Welch Allyn is indicated to measure the temperature of adult and pediatric patients.
With the addition of noninvasive blood pressure and temperature to the Root platform, clinicians can easily and automatically chart vital signs data directly from Root. Enhancing the solution with Masimo Patient SafetyNet™ allows data to be sent directly to the patient's electronic medical record (EMR) while alarms and alerts are seamlessly forwarded to the patient's clinician.
Root with noninvasive blood pressure and temperature provides a monitoring solution for step-down, medical, and surgical environments. Clinicians on a unit equipped with this technology are expected to be able to continuously monitor their patients and automatically document vital signs while improving workflow, allowing them to spend more time with their patients.
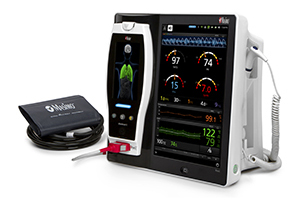
Masimo Root® with noninvasive blood pressure and temperature
Root offers a high visibility display with intuitive, touchscreen navigation for easy and adaptable use in any hospital environment:
- Integrates measurements from Masimo Radical-7® handheld for SET® Measure-through Motion and Low Perfusion™ pulse oximetry, continuous hemoglobin (SpHb®), PVi®, and other noninvasive rainbow® parameters, or the Radius-7™ patient-worn monitor for continuous pulse oximetry and respiration rate monitoring of mobile patients.
- Flexible measurement expansion through Masimo Open Connect™ (MOC-9™), including:
> ISA™ CO2 capnography for sidestream monitoring featuring Nomoline™ technology, allowing for the cost-effective use of consumables.
- Additional MOC-9 modules available include measurements such as SedLine® brain function monitoring.
- Automatic display of parameters, waveforms, and viewing configuration based on the clinician's presence through MyView™ technology enabled with Patient SafetyNet™ version 5000 or higher.
- Optional USB barcode scanner for easy patient association.
- Available roll stand for ease of transport throughout the hospital.
- Built-in wireless radio allows data to be transmitted continuously during rounds.
"We are excited to bring this addition to the U.S. market," said Joe Kiani, Founder and CEO of Masimo. "We designed Root to do for patient monitoring what PCs did for computing. By creating a platform which allows third-party measurement expansion, with an intuitive user interface and network connectivity, we hope to speed up innovation while reducing the cost of monitoring, as well as potentially improving patient care and patient safety."
For more information on Root with noninvasive blood pressure and temperature, go to www.masimo.com.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive open architecture patient monitoring and connectivity platform designed to speed the pace of innovation and reduce the cost of care. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo's Root® with noninvasive blood pressure and temperature. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Root® with noninvasive blood pressure and temperature, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo's rainbow® Acoustic Monitoring (RAM™) was Associated with Fewer Alarm Events During Planned Moderate Sedation in New Clinical Study
For Breath Rates Less Than or Equal to 4 Breaths Per Minute, RAM Showed 78% True Positives and 22% False Positives, While Capnography Showed 30% True Positives and 70% False Positives
Irvine, California – February 12, 2016 – Masimo (NASDAQ: MASI) announced today that Masimo's rainbow® Acoustic Monitoring (RAM™) was associated with fewer alarm events during planned moderate sedation in a new clinical study.1
This two-part pilot study used RAM and SedLine® brain function monitoring as the additional monitoring parameters during planned moderate sedation to (1) determine the incidences of alarm events (desaturation, respiratory depression and deeper than intended sedation), and (2) determine whether monitoring with these parameters is associated with fewer alarm events.
RAM noninvasively and continuously measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer that is applied to the patient's neck.
In the two-part study, published in the journal Anesthesia & Analgesia by Dr. Richard Applegate II and colleagues from the Loma Linda University School of Medicine, data were collected from adult patients undergoing gastroenterology or interventional radiology procedures under moderate sedation. The patients were monitored per standard protocols (electrocardiography, blood pressure, pulse oximetry, and capnography) along with the additional parameters RAM and SedLine. RAM and SedLine were not visible to the care teams in part one of the study (standard) but were visible to the care teams in part two of the study (advanced). Alarm events were defined as desaturation (SpO2 <=92%), acoustic respiration rate <=8 breaths per minute, and PSi <=50.
Data were analyzed from 90 patients (44 standard and 46 advanced). There were 55% fewer alarm events in advanced patients compared with standard patients (median 2.5 vs. 5.5, p=0.038). Fewer advanced patients had >=1 respiratory depression event (17 vs 26; p=0.035) or >=1 desaturation event (15 vs 25; p=0.02).
The investigators also compared respiratory rate events between capnography (using the Covidien Capnostream™ 20p) and RAM in the standard group and conducted a retrospective analysis to determine the true positive rates for capnography and RAM. The true positive rates were 30% and 78% respectively; the false positive rates were 70% and 22% respectively.
There were no significant differences between groups in the number of deeper than intended sedation events using SedLine.
The authors concluded that "the use of advanced monitoring during planned moderate sedation was associated with fewer alarm events, patients experiencing desaturation, and patients experiencing respiratory depression alarm events. ... The findings The findings of this pilot study suggest that further study into the safety and outcome impacts of advanced monitoring during procedure-related sedation is warranted."
For more information on RAM and SedLine, go to www.masimo.com.
References
1 Applegate RL II, Lenart J, Malkin M, Meineke MN, Qoshlli S, Neumann M, Jacobson JP, Kruger A, Ching J, Hassanian M, Um M. Advanced Monitoring Is Associated with Fewer Alarm Events During Planned Moderate Procedure-Related Sedation: A 2-Part Pilot Trial. Anesth Analg. 2016 Feb 1. [Epub ahead of print].
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive open architecture patient monitoring and connectivity platform designed to speed the pace of innovation and reduce the cost of care. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo's rainbow® Acoustic Monitoring (RAM™) and SedLine® brain function monitoring technologies. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Root® with SedLine® and RAM™, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


In New Clinical Study Masimo's Oxygen Reserve Index Helps Clinicians Detect Impending Desaturation in Pediatric Patients
Neuchatel, Switzerland – February 4, 2016 – Masimo (NASDAQ: MASI) announced today that in a new clinical study, Masimo's Oxygen Reserve Index (ORI™), the first noninvasive and continuous parameter to provide insight into patients' oxygen reserve when they are receiving supplemental oxygen, helped clinicians in the early identification of impending desaturation in pediatric patients during induction of anesthesia.1
Oxygen saturation (SpO2) using pulse oximetry provides noninvasive, continuous visibility to arterial blood oxygenation in hypoxia (lower than normal oxygenation) and normoxia (normal oxygenation). For procedures such as induction of anesthesia and intubation, clinicians will usually build an "oxygen reserve" in the patient by providing supplemental oxygen or "pre-oxygenation," creating a transitional state of hyperoxia (higher than normal oxygenation). The extent of hyperoxia can be determined by the measured partial pressure of oxygen in the blood (PaO2 ), which requires invasive arterial blood sampling and laboratory analysis. When patients have low oxygen reserve prior to intubation of the trachea, they are susceptible to sudden hypoxia, depending upon the time taken to perform the intubation.
ORI, Masimo's 11th rainbow® parameter2, is a relative index of the partial pressure of oxygen in arterial blood (PaO2) within the range of 100 to 200 mmHg that can provide insight into significant changes in oxygen reserve. ORI is intended to supplement, not replace, SpO2 monitoring and PaO2 measurements.
In a prospective study published in Anesthesiology and conducted at Children's Medical Center in Dallas, Texas, Dr. Peter Szmuk and colleagues evaluated whether ORI could provide a clinically important warning of impending desaturation in pediatric patients with induced apnea after pre-oxygenation. The investigators enrolled 33 patients in this study. Eight of these resumed spontaneous ventilation during the study period, leaving 25 apneic patients to evaluate, with an average age of 7.6 years. Data were recorded continuously by the Masimo Radical-7® Pulse CO-Oximeter®, connected to the patient through an R1 25L rainbow® sensor. ORI was retrospectively calculated and was not visible to investigators.
After pre-oxygenation with supplemental oxygen, anesthesia induction, and endotracheal intubation, the anesthesia circuit was disconnected and SpO2 was allowed to decrease to 90% before ventilation recommenced. During the period of apnea, the ORI progressively decreased from a mean of 0.73 ±0.2 at the beginning of apnea to 0.37 ± 0.1. The SpO2 remained at 100% during this initial period. After decreases in ORI that would trigger the ORI alarm if monitored in real time, there was a median elapsed time of 31.5 seconds before the SpO2 decreased to 98%. After reinstitution of ventilation, SpO2 values declined further to 88%, before recovering to 98%, 34 seconds later. The researchers concluded that "In this pilot study, we found that during prolonged apnea in healthy anesthetized children, the ORI detected impending desaturation in median of 31.5 seconds (IQR, 19 to 34.3 seconds) before noticeable changes in SpO2 occurred. Knowing even roughly how much time remains before the rapid desaturation phase begins seems likely to guide proper decisions."
Radical-7® with Root® has a CE Mark with the ORI parameter. ORI is not FDA cleared and is not available for sale in the United States.
For more information on ORI go to http://www.masimo.co.uk/ori/.
References
1 Szmuk P, Steiner JW, Olomu, PN, Ploski, RP, Sessler, DI, Ezri, T. Oxygen Reserve Index A Novel Noninvasive Measure of Oxygen Reserve—A Pilot Study. Anesthesiology 2016; 124:00-00. doi:10.1097/ALN.0000000000001009.
2 11 parameters include: 1) oxygen saturation (SpO2); 2) Pulse rate; 3) Perfusion index (Pi); 4) Pleth Variability Index (PVi®); 5) Respiration Rate from the pleth (RRp®); 6) Total hemoglobin (SpHb®); 7) Oxygen Content (SpOC™); 8) Carboxyhemoglobin (SpCO®); 9) Methemoglobin (SpMet®); 10) Fractional oxygen saturation (SpfO2®); 11) Oxygen Reserve Index (ORI™).
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive open architecture patient monitoring and connectivity platform designed to speed the pace of innovation and reduce the cost of care. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo's Oxygen Reserve Index (ORI™). These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including ORI™, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo to Report Fourth Quarter 2015 Financial Results after Market Close on Tuesday, February 23
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., January 29, 2016 – Masimo (NASDAQ: MASI) announced today that it will release financial results for the fourth quarter ended January 2, 2016, after the market closes on Tuesday, February 23, 2016. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 40429223. After the live webcast, the call will be available on Masimo's website through March 22, 2016. In addition, a telephonic replay of the call will be available through March 8, 2016. The replay dial-in numbers are (855) 859-2056 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 40429223.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive open architecture patient monitoring and connectivity platform designed to speed the pace of innovation and reduce the cost of care. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Eli Kammerman
Masimo
Phone: (949) 297-7077
Email: ekammerman@masimo.com
Media Contact:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


New Study Shows Utility of Masimo rainbow Acoustic Monitoring™
IRVINE, California – January 21, 2016 – Masimo (NASDAQ: MASI) announced today that in a new study that compared acoustic respiration rate (RRa®) from Masimo's rainbow® Acoustic Monitoring (RAM™) to respiration rate from conventional capnography, researchers concluded that RAM was useful for monitoring dental patients under intravenous anesthesia.1
rainbow Acoustic Monitoring (RAM) from Masimo.
The Anesthesia Patient Safety Foundation (APSF) and The Joint Commission recommend continuous oxygenation and ventilation monitoring in all patients receiving opioid-based pain medications.2 RAM noninvasively and continuously measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck.
The study, published in the Journal of Clinical Monitoring and Computing by Dr. Kentaro Ouchi and colleagues from Japan, compared the respiration rate measurements from RAM and capnography (Psychorich IS nasal cannula and BP-608 Omron Colin monitor) in non-intubated patients with dental anxiety undergoing dental treatment. A total of 1953 data points of respiratory rate were taken from the start to the end of anesthesia. Over the entire observation period, the results showed a significantly higher detection of respiratory rate by RAM (1884 points, 96.5%) than by capnography When comparing respiration rate from RAM and capnography during the induction, intraoperative, and emergence periods, the 95% limits of agreement (LOA) were -7.4 to 6.7, -5.4 to 7.0, -3.9 to 5.3, respectively. The researchers commented that this study was only a comparison between two devices, since neither method could be considered a "corrective control," or gold-standard reference.
The authors concluded, "In comparison between capnography using a nasal cannula for continuous monitoring for the respiratory rate, the acoustic method is useful during intravenous general anesthesia in unintubated spontaneously breathing patients undergoing dental procedures." The authors note that the acoustic method might not accurately detect respiratory rate in cases in which a dental air turbine is used.
For more information on the RAM Acoustic Respiratory Rate Monitoring System, go to www.masimo.com.
References
1 Ouchi (et al.), Acoustic Method Respiratory Rate Monitoring is Useful in Patients Under Intravenous Anesthesia. J Clin Monit Comput 2016; published online.
2 Stoelting, RK et al. APSF newsletter. 2011. www.apsf.org.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive open architecture patient monitoring and connectivity platform designed to speed the pace of innovation and reduce the cost of care. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results or studies; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo's rainbow® Acoustic Monitoring (RAM™) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation, including the dental setting; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Dr. Steven Barker Awarded the Society for Technology in Anesthesia's J.S. Gravenstein Award for Lifetime Achievement
IRVINE, California – January 19, 2016 – Masimo (NASDAQ: MASI) announced today that a member of its Board of Directors, Chairman of its Scientific Advisory Board, and its Chief Science Officer, Steven Barker, Ph.D., M.D., was honored with the 2016 J.S. Gravenstein Award recognizing lifetime achievement at this month's Society for Technology in Anesthesia (STA) Annual Meeting in Palm Beach, Florida.
"We selected Dr. Barker as the recipient of the Gravenstein Award because of his years of innovation, instruction and service," said Dr. Joseph Orr, President of the STA. "Dr. Barker is a rare combination of training in the physical sciences (Ph.D. in aerospace engineering) and in medicine. Over the years, he has been able to apply his training, research and talent to study and improve medical monitoring devices. Through his collaboration with Masimo, Dr. Barker's work has improved technology available for clinical anesthesia practice."
The STA was founded in 1988 and is an international organization of physicians, engineers, students and others with an interest in anesthesia-related technologies. The organization also publishes the journal Anesthesia & Analgesia. Each year, the STA bestows the J.S. Gravenstein Award for lifetime achievement in the area of technology in anesthesia. The J.S. Gravenstein Award is named for J.S. "Nik" Gravenstein (1925-2009), a founding member and former president of the STA. For over thirty years, Dr. Gravenstein was a driving force in advancing anesthesia technology, patient simulation and anesthesia patient safety.
"This is a great honor and we are very proud of Steve," said Joe Kiani, Founder and CEO of Masimo. "He has always been at the forefront of technology development and research in every area of anesthesia, but what sets Steve apart is his commitment to the truth and integrity. His work has contributed to improving quality of care and patient safety around the world."
Steven J. Barker, Ph.D., M.D., received his Ph.D. in Aeronautical Engineering from the California Institute of Technology in 1972, and his M.D. from the University of Miami in 1981. He has reached the rank of tenured professor in both engineering (University of California at Los Angeles) and anesthesiology (University of California at Irvine, University of Arizona). He chaired the Department of Anesthesiology at UC Irvine from 1990 to 1995, and then at the University of Arizona from 1995 to 2013. He has published over 150 scholarly works, including 15 textbook chapters. Dr. Barker has served as president of the national organization of anesthesiology department chairs (AAPD) and of the Society for Technology in Anesthesia (STA), senior oral examiner for the American Board of Anesthesiology, and Section Editor for Technology for the journal Anesthesia & Analgesia. During his 18-year tenure as anesthesiology chair at the University of Arizona, Dr. Barker was active in a number of governance roles outside of his department. He also served seven years as the American Society of Anesthesiologists (ASA) Director for Academic Anesthesiology, representing all university anesthesiology departments for ASA. Since 2005, Dr. Barker has been a member of Masimo's Board of Directors and Chairman of Masimo's Scientific Advisory Board. In 2015, Dr. Barker also took on the role of Chief Science Officer of Masimo.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive open architecture patient monitoring and connectivity platform designed to speed the pace of innovation and reduce the cost of care. Masimo is also taking an active leadership role in mHealth with products such as the Radius-7™ wearable patient monitor and the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Media Contacts:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com


North Carolina's Appalachian Regional Healthcare System Selects Patient SafetyNet™ and Radius-7™
Irvine, California and Boone, North Carolina – January 13, 2016 - Masimo (NASDAQ: MASI) and Appalachian Regional Healthcare System announced that Watauga Medical Center in Boone is the first hospital in North Carolina to install Masimo Patient SafetyNet, a remote monitoring and clinician notification system, and Radius-7, a patient-worn monitor that is designed to allow for patient mobility along with continuous monitoring.
"We are always looking for cutting-edge technologies focused on patient safety," said Richard Sparks, President & CEO, Appalachian Regional Healthcare System. "We wanted a system that provides continuous monitoring that wasn't restricted to the patient's room. The Radius-7 combined with Patient SafetyNet allows us the ability to keep a close eye on our patients 24/7."
Continuous patient surveillance with Patient SafetyNet has been shown to reduce the need for rescue events and intensive care unit transfers in hospitals and, as a result, can reduce costs related to these events.1,2 When changes occur in the measured values, which may indicate deterioration in the patient's condition, in addition to bedside alerts, the alert also shows in the Telemetry room and nurse's stations. This allows the telemetry technician to contact the unit where the alarm is happening immediately. Masimo Patient SafetyNet works in conjunction with Masimo beside monitors and the Radius-7 wearable monitor to provide wireless, continuous and non-invasive monitoring of oxygenation, pulse rate and respiration, among other parameters.
Appalachian Regional Healthcare System's Charles A. Cannon, Jr. Memorial Hospital also recently implemented continuous monitoring. At this location, the monitors are connected to the patient call system. When an alert goes off, it sounds through the call lights and then sends a message to the nurse's station call monitor.
"Watauga Medical Center in Boone and Cannon Memorial Hospital in Linville understand the importance of patient safety," said Joe Kiani, Founder and CEO of Masimo. "We are excited to see more and more hospitals implement continuous monitoring of their patients in post-surgical wards, as part of their patient safety protocols."
1 Taenzer A.H., Pyke J.B., McGrath S.P., Blike G.T. Anesthesiology. 2010 Feb;112(2):282-7.
2 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring - The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online.
About Appalachian Regional Healthcare System
Appalachian Regional Healthcare System (ARHS), the healthcare leader in the High Country, is comprised of two hospitals - Charles A. Cannon, Jr. Memorial Hospital in Linville and Watauga Medical Center in Boone - a physician practice management group - Appalachian Regional Medical Associates (ARMA) - the Blowing Rock Rehabilitation & Davant Extended Care Center in Blowing Rock - and Appalachian Regional Healthcare Foundation. Appalachian Regional Healthcare System stays committed to promoting health in the High Country, enhancing quality of life and simply "making life better." This charge makes Appalachian Regional Healthcare System the premier healthcare system in this region. For more information, visit www.apprhs.org.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform designed to speed the pace of innovation and reduce the cost of care. Masimo is also taking an active leadership role in mHealth with products such as the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Gillian Baker
Watauga Medical Center
Phone: (828) 268-8958
Email: gbaker@apprhs.org
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.


Masimo to Debut Respiration Rate in MightySat™ at Consumer Electronics Show – First Fingertip Pulse Oximeter to Measure Breath Rate
Visit CES booth 73710 for Live Audience Cycling Competition
Irvine, California – January 6, 2016 – Masimo (NASDAQ: MASI) announced today that it has added a new parameter, respiration rate from the pleth (RRp™), to its MightySat fingertip pulse oximeter. MightySat is intended for general wellness and health applications including sports, fitness, and relaxation management.
Respiration rate, or the number of breaths per minute, is infrequently measured outside of healthcare settings because it typically requires manual counting of breaths with a timer and then multiplying to convert to a per minute rate or chest leads or straps that are not convenient to use. Fingertip pulse oximeters are being increasingly used for general wellness and health applications. Now consumers will be able to quickly and easily measure respiration rate, in addition to arterial oxygen saturation (SpO2), pulse rate (PR), perfusion index (Pi), and Plethysmograph Variability Index (PVi®).
Masimo pulse oximeters are #1 in U.S. hospitals1 because they provide accurate measurements when other pulse oximeters fail, by using a revolutionary invention called Signal Extraction Technology (SET®). MightySat features hospital-grade SET pulse oximetry and also provides parameters not available on other fingertip pulse oximeters, such as RRp and PVi. In addition, MightySat offers a Bluetooth wireless interface to the Masimo Personal Health and Apple Health App to track, trend, and communicate measurements. MightySat is increasingly being used by elite athletes and teams to assess and improve performance.
"The addition of respiration rate to the MightySat is a game changer because we can now quantify breathing - a fundamental indicator of health, wellness, and sports performance," stated Olympic Silver Medalist Dotsie Bausch.
"Sometimes matches are won by a few points, and I need every breath to count," said Coco Vandeweghe, Professional Tennis Player on the WTA Tour. "MightySat makes breath tracking easier."
"We believe the MightySat will help transform the wearables market by providing fitness enthusiasts with the same valuable personal data that to date has only been available to elite athletes," said Joe Kiani, Chairman and CEO of Masimo.
"Helping athletes to improve their overall performance ecosystem is what I do," stated Tim DiFrancesco, PT, DPT, ATC, CSCS, Head Strength and Conditioning Coach for the Los Angeles Lakers. "That requires gaining insight into the well-being of the person as well as the physiology within the person. Many objective monitoring tools fail to provide accurate and valuable information in a swift and noninvasive manner, but MightySat succeeds. After incorporating MightySat into my athlete monitoring toolbox, I see an impressive list of potential value through quantifying and measuring markers that give a more granular look at athlete readiness, fatigue/overtraining, fuel source utilization, and cardiovascular conditioning."
This year at the Sands Expo in Las Vegas, attendees can visit Masimo at CES booth 73710 and observe MightySat measurements before and after a live audience cycling competition. Cycling participants will be aided by two experts, Stig Severinsen and Dotsie Bausch. Stig is a four-time World Champion freediver and holds multiple Guinness World Records, including history's longest breath-hold, of 22 minutes. Dotsie is a seven-time USA Cycling National Champion, two-time Pan-American Champion, and silver medalist in team pursuit cycling at the 2012 London Olympics. Both Stig and Dotsie will also be available in the booth to answer questions about how they have incorporated MightySat into their health and fitness routines.
MightySat is available for purchase on Amazon U.S. and Amazon U.K. MightySat with Respiration Rate will be available for purchase by the end of February 2016.
1 iData Research. U.S. Market for Patient Monitoring Equipment. 2014.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and more recently, Pleth Variability Index (PVi®) and Oxygen Reserve Index (ORI™), in addition to SpO2, pulse rate, and perfusion index (Pi). In 2014, Masimo introduced Root®, an intuitive patient monitoring and connectivity platform designed to speed the pace of innovation and reduce the cost of care. Masimo is also taking an active leadership role in mHealth with products such as the MightySat™ fingertip pulse oximeter. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, those related to the expected adoption of MightySat. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVi are trademarks or registered trademarks of Masimo.
