News & Media-2010
Home / 2010
2010
NEWS & MEDIA:

Happy Holidays from Masimo
Annual Livewire Charity
Irvine, California – December 23, 2010 –It is at this very special time of the year that we look forward to sharing our blessings with others. By sending us an email with your charity preference from the list below, you can choose whom we make a distribution to.
It is with great appreciation for our customers, partners, shareholders and employees that we close out this year by making a donation of $10 to the charity of your choice. We will make this donation in the name of each person who is an official member of Livewire as of today and who responds to this Livewire with his or her charity choice from the list below:

- Amnesty International
- CARE
- Doctors Without Borders
- Huntington's Disease Society of America
- Make-a-Wish Foundation
- March of Dimes
- Masimo Foundation for Ethics, Innovation, and Competition in Healthcare
- Opportunity International
- SOS Children's Villages
- Swan Foundation
- UNICEF
- United Way
- World Vision
Please send us an e-mail specifying your selection to: charity@masimo.com. Only requests by e-mail to this address will be processed. We also encourage you to include any comments or suggestions you might have that will help us to better fulfill our mission and adhere to our guiding principles.
We are looking forward to a new year filled with joy, good health and peace. A 2011 rich with unlimited possibilities and innovation. We are excited that we have launched the Masimo Foundation for Ethics, Innovation and Competition in Healthcare dedicated to improving patient safety and delivering advanced healthcare to people worldwide who may not otherwise have access to lifesaving technologies. In this new year, the foundation will be offering grants and we hope you will send any ideas or deserving projects our way. Together we can save lives and improve the world. Please visit the website at www.masimofoundation.org.
We thank each of you for your support in helping us to make life better for the clinicians and patients we diligently serve, and look forward to a new year full of great possibilities.
With sincere gratitude and warmest regards, 
Masimo Mission Statement
Improving patient outcomes and reducing cost of care by taking noninvasive monitoring to new sites and applications.
Masimo Guiding Principles
- Remain faithful to your promises and responsibilities.
- Thrive on fascination and accomplishment and not on greed and power.
- Make each day as fun as possible.
- Strive to make each year better than the year before, both personally and for the Team.
- Do what is best for patient care.


Masimo to Present at 29th Annual J.P. Morgan Healthcare Conference
IRVINE, Calif., December 22, 2010 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the 29th Annual J.P. Morgan Healthcare Conference at the Westin St. Francis Hotel in San Francisco on Tuesday, January 11, 2011, at 11:30 a.m. PT. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


New Clinical Study Presented at the American Association of Respiratory Care Finds Masimo Rainbow Acoustic Monitoring Accurate for Respiration Rate Assessment
Masimo Also Awarded Prestigious Zenith Award for Third Straight Year
Irvine, California – December 13, 2010 – Masimo (NASDAQ: MASI) announced today that a new clinical study demonstrating the accuracy of its latest medical technology breakthrough—Rainbow Acoustic Monitoring™—was presented last week at the American Association of Respiratory Care's (AARC) 56th Annual International Respiratory Congress in Las Vegas. The largest gathering of respiratory care clinicians in the world, the AARC Annual Meeting provides an important platform for previewing the latest in medical developments and clinical research to respiratory care thought-leaders.
The study presented by Jim Kumpula, RRT, System-wide Manager of Respiratory Care Services at Swedish Medical Center, evaluated the accuracy of acoustic respiration rate (RRa) measurements, obtained noninvasively with Masimo's Rainbow Acoustic Sensor and continuously displayed on the Radical-7 Pulse CO-Oximeter, in 25 patients on an acute nursing unit in comparison to manual estimates of respiration rate obtained via clinical auscultation (listening to breathing sounds with a stethoscope) and visual observations of patient breathing. After analyzing 187 respiratory rate data pairs collected (7.4 +/- 4.6 data pairs per patient) with bias and precision of 0.8 and 3.4 bpm respectively, Kumpula concluded that Rainbow Acoustic Monitoring was a "simple and automatic method of measuring respiration rate at the bedside, with clinically acceptable accuracy" that could be of "significant value in a wide range of clinical settings including the general care floor, PACU, OR, sleep laboratories, and any care area utilizing conscious sedation."1
Respiration rate is an important vital sign and is especially important for post-surgical patients receiving patient-controlled analgesia (PCA) for pain management as sedation can induce respiratory depression and place patients at considerable risk of serious injury or death.2-5 Although the Anesthesia Patient Safety Foundation (APSF) recommends continuous oxygenation and ventilation monitoring in all patients receiving opioids,6-7 current methods for respiration rate monitoring are limited by reliability or patient tolerance. Early this year Swedish Medical Center became a limited market release site for Masimo's Rainbow Acoustic Monitoring technology, which according to Kumpula has been beneficial.
According to Dr. Michael O'Reilly, Chief Medical Officer at Masimo, "Rainbow Acoustic Monitoring technology provides a new cost-effective solution to address the longstanding and growing need to more accurately and easily monitor patient breathing. This study, from the Swedish Medical Center, underscores the value and impact of this new medical technology tool for significantly improving patient safety and decreasing the cost of care."
Additionally, underscoring the Company's commitment, dedication, and industry leadership in providing best-in-breed, innovative patient care solutions, Masimo received its third consecutive Zenith Award. As the AARC's top industry honor for quality and service excellence, more than 400 companies were eligible for one of five Zenith Award honors. Award winners are chosen by the association's membership—more than 44,000 respiratory care professionals—based upon the quality of the company's equipment and/or supplies, responsiveness, service record, truth in advertising, accessibility and helpfulness of sales personnel, and overall support of the respiratory care profession.
Joe Kiani, Founder and CEO of Masimo, stated, "Confronted with the challenges of delayed lab results, patient tolerance/compliance issues, and time-consuming invasive procedures, respiratory clinicians must provide vital, life-sustaining care for the critically-ill in the face of enormous clinical uncertainty. It is gratifying to see, through studies like this one from Swedish Medical Center, that our noninvasive products and innovative technologies are providing respiratory care clinicians with the immediate access to critical physiological measurements, detailed clinical data, and, in turn, earlier detection of adverse respiratory conditions they need to improve clinical care and patient outcomes. And, we are honored by the appreciation of so many AARC members who have once again recognized us with the Zenith Award for the superior quality and performance of our products and the service, commitment, and integrity excellence of our company."
1 Kumpula, J., Harrison, R. "Accuracy of Acoustic Respiration Rate Monitoring in an Acute Nursing Unit." Swedish Medical Center, Seattle, Washington. AARC abstract 811730 presented December 9, 2010, (Open Forum #15—Monitoring/Equipment Part II 9:30-11:25am).
2 Joint Commission on Accreditation of Healthcare Organizations. "Sentinel Event Alert: Patient Controlled Analgesia By Proxy." Chicago: JCAHO, 2004
3 Institute for Safe Medication Practice. "Safety Issues with Patient-Controlled Analgesia: Part 1-How Errors Occur." Huntingdon Valley: ISMP, 2003
4 Institute for Safe Medication Practices. "Safety Issues with Patient-Controlled Analgesia: Part II – How to Prevent Errors." Huntingdon: ISMP, 2003
5 Bird M. "Acute Pain Management: a New Area of Liability for Anesthesiologists." ASA Newsletter. Park Ridge: American Society of Anesthesiologists, 2007
6 Weinger MB. "Dangers of Postoperative Opioids: APSF Workshop and Whitepaper Address Prevention of Postoperative Respiratory Complications." APSF Newsletter, 2006;21:61–7. For further reading, visit: http://www.apsf.org/newsletters/html/2007/winter/01_opioids.htm.
7 Stoelting RK, Weinger MB. "Dangers of Postoperative Opioids—Is There a Cure?" APSF Newsletter, 2009;24:25-26. For further reading, visit: http://www.apsf.org/newsletters/html/2009/summer/01_opiods.htm.
8 Macknet, M., Allard, M., Kimball-Jones, P., Rook, J., Applegate, R. "Accuracy of Respiratory Rate Using an Acoustic Respiration Monitor." Loma Linda University, Loma Linda, California.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care...by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, Pulse CO-Oximetry, Pulse CO-Oximeter, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pronto-7, Pronto, Patient SafetyNet, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Global Education Campaign Launched to Warn FireFighters and First Responders of Occupational Hazards of "The Silent Killer
Spotlights the Health Consequences of Unsuspected Carbon Monoxide (CO) Poisoning During Fire Operations and Urges FireFighter/First Responder Protection and Safety
Irvine, California – December 2, 2010 – Masimo (NASDAQ: MASI), the International Association of FireFighters (IAFF), and the International Association of Fire Chiefs (IAFC), jointly announced today the launch of "The Silent Killer" educational campaign aimed at raising awareness of the duty-related dangers of carbon monoxide (CO) poisoning and reducing the known risk factors that unnecessarily kill or injure firefighters each year. This important new health and safety campaign includes a dramatic six-minute video that highlights the immediate and long-term health risks associated with CO exposure, the emotional impact these risks can have on firefighters and their families and advocates proper prevention strategies. The video can be viewed online at www.thesilentkiller.net and DVDs will be widely distributed to fire departments throughout the world.
Carbon monoxide poisoning is a danger at every fire, but this "Silent Killer" is often present without symptoms, making awareness, proper diagnosis and treatment difficult.1 This puts firefighters on the scene of a fire at significant risk because even mild CO poisoning can rob the brain of oxygen,2 which can lead to poor decision making.3 It can also rob the heart, brain, and vital organs of oxygen, causing life-threatening complications — with half of on-duty firefighter deaths being attributed to heart attacks or stroke.4 Just one severe CO poisoning almost doubles the long-term risk of death.5
This new education campaign urges firefighters to take personal responsibility for their health and safety by recognizing the occupational hazards of CO exposure and wearing protective masks during both active fire and overhaul operations to prevent unnecessary risks. It also encourages firefighters to get their CO levels tested on the fire scene with an approved noninvasive portable device6 and, if elevated, to seek immediate treatment, even if they are feeling well.
The video was produced and narrated by Randolph Mantooth, widely recognized for his portrayal of Los Angeles County FireFighter/Paramedic "Johnny Gage" in the popular 1970s NBC Universal television series "Emergency!" "Too many of our fire rescuers and first responders do not believe they too are in danger and, consequently, do not adequately protect themselves from becoming unknowing victims of CO poisoning," said Mantooth, a vocal advocate for CO awareness and prevention since his own near-death CO experience 20 years ago, "That's why it has become my mission to ensure that each and every firefighter and emergency first responder knows and understands these significant occupational hazards, how to properly protect themselves, and how to prevent unnecessary health risks to improve the odds that they will be around tomorrow to do what they were born to do," Mantooth said.
"CO is a significant and deadly occupational risk factor for firefighters," said Harold A. Schaitberger, General President of the International Association of FireFighters. "We know that carbon monoxide (CO) is present in every fire and symptoms of CO poisoning are nonspecific and easy to miss. Any firefighter potentially exposed to CO and presenting with headache, nausea, shortness of breath or gastrointestinal symptoms should be assessed."
IAFC President and Chairman of the Board, Chief Jack Parow, stated: "We are pleased to be a part of this important educational campaign. Carbon monoxide is a real risk that firefighters and first responders face every day. With the proper awareness, precautions, and testing, we can prevent firefighter deaths and extend lives." The IAFC will be assisting with distribution of the video, including sending complimentary copies of the video to its membership. "As with many issues, education is the key to success," continued Parow.
Masimo Founder and CEO, Joe Kiani, stated, "We are proud to have supported the development and dissemination of this educational material for firefighters and first responders. The 'Silent Killer' campaign is our opportunity to help save the lives of the men and women who put their lives on the line every day for each of us. We know that the more successful this campaign is in reaching and educating them about the hidden dangers and risks of carbon monoxide poisoning, the fewer of our heroes will become victims and have their lives shortened by carbon monoxide poisoning."
The new website, www.thesilentkiller.net was specifically developed to help raise awareness among firefighters, first responders, emergency medical service (EMS) personnel, and their friends and families about the unsuspected occupational dangers of CO poisoning and how best to protect themselves. Designed as a destination site for all emergency first responders, the "Silent Killer" website features the new video along with a host of other important health and safety resources, including:
- CO Fact Sheets with medical/scientific information about the health and safety risks of CO poisoning
- Special CO supplement published in the Journal of Emergency Medical Servicestitled "The Silent Killer: CO Monitoring Adds a New Dimension to Firefighter Rehab & Emergency Care"
- CO screening and medical monitoring outlined in NFPA 1584: National Standard on the Rehabilitation Process for Members During Emergency Operations and Training Exercises
- Links to other firefighter, first responder, and EMS industry websites and resources
- Free online ordering of "The Silent Killer" DVD, as well as other available resources
1 Hampson, NB, et al: "Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture?" American Journal of Emergency Medicine. 26:665-669, 2008.
2 Bledsoe, BE: "The Perils of CO" FireRescue Magazine. September 2005.
3 Jakubowski, G. The Invisible Incidents: How to respond to CO alarms. FireRescue Magazine. 22(11):52–55, 2004.
4 Bledsoe, BE. "The Dangers of CO: Understanding Cardiovascular Risks to Responders from CO Exposure." Journal of Emergency Medical Service. 32:54-59, 2007.
5 Hampson, NB et al. "Increased long term mortality among survivors of acute carbon monoxide poisoning." Crit Care Med. 2009; 37(6): 1941-47.
6 NFPA 1584: Standards on the Rehabilitation Process for Members During Emergency Operations and Training Exercise. Annex A section A.6.2.6.4(1)
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
About the International Association of FireFighters
The International Association of FireFighters, headquartered in Washington, DC, represents more than 298,000 full-time professional firefighters and paramedics and is the leading advocate for health and safety of first responders in North America. More information is available at www.iaff.org
About the International Association of Fire Chiefs
The IAFC represents the leadership of firefighters and emergency responders worldwide. IAFC members are the world's leading experts in firefighting, emergency medical services, terrorism response, hazardous materials spills, natural disasters, search and rescue, and public safety legislation. Since 1873, the IAFC has provided a forum for its members to exchange ideas, develop professionally and uncover the latest products and services available to first responders.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: :risks related to assumptions regarding repeatability of clinical study results; risks related to the belief that the Masimo Rad-57 will allow clinicians to noninvasively and immediately measure the amount of CO in the bloodstream for all patients and under all conditions; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
Phone: (949)297-7348
Email: dbanks@masimo.com
Ann Davison
IAFC
Phone: (703)537-4829
Email: adavison@iafc.org
Tim Burn
IAFC
Phone: (202)824-1566
Email: tburn@iaff.org
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Board of Directors Declares Special Cash Dividend
Dividend Set at $0.75 Per Share
Irvine, California – November 22, 2010 – Masimo (NASDAQ: MASI) announced today that its Board of Directors has declared a special $0.75 per share cash dividend, payable on December 21, 2010 to stockholders of record as of the close of business on December 7, 2010. The total dividend payout is expected to be approximately $44.3 million, based on the current shares outstanding and will be the third instance of a special dividend paid by Masimo in the past four years.
Masimo Founder, CEO and Chairman of the Board, Joe Kiani, stated, "The special dividend is another step in demonstrating our commitment to enhancing stockholder value. The Board's decision to pay a dividend reflects our strong balance sheet, confidence in our long term outlook and capital structure. In addition, due to the uncertainty over potential changes in tax policy, the timing of this dividend will allow Masimo stockholders to take advantage of the current low dividend tax rate."
While dividends are not routine for the company, the company has previously paid special dividends to shareholders totaling $4.09 per share related to the 2006 intellectual property patent suit settlement agreement with a competitor and $2.00 per share in March 2010. While there can be no guarantees of future dividends, the Masimo Board remains committed to enhancing stockholder value based on its consideration of various factors, including the company's operating results, financial condition and anticipated capital requirements.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate (PR), and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo's rainbow SET Pulse CO-Oximetry technology will provide sufficient sensitivity and specificity to measure SpHb, SpOC, PVI, SpO2, SpCO, SpMet, PI, PVI and PR in real-time for all patients, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Investor Contact:
Sheree Aronson
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at Piper Jaffray 22nd Annual Healthcare Conference
IRVINE, Calif., November 19, 2010 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Piper Jaffray 22nd Annual Healthcare Conference at The New York Palace in New York City on Tuesday, November 30, at 12:30 p.m. ET. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Prime Healthcare Converts System-wide to Masimo rainbow SET Technology
13-Hospital Integrated Healthcare System in California Standardizes Patient Care to New Noninvasive Monitoring Platform
Irvine, California – November 18, 2010 – Prime HealthcareandMasimo (NASDAQ: MASI) today jointly announce the system-wide conversion of Prime Healthcare's hospitals to Masimo rainbow® SET technology. The system-wide conversion ensures that patients visiting any Prime Healthcare hospital in California will be cared for using the most technologically and clinically-advanced oximetry and noninvasive patient monitoring solutions available.
"We're pleased and quite amazed with our conversion to Masimo," stated George Garcia, Regional Director of Cardiopulmonary Services at Prime Healthcare. "Not only is the technology impressive in that it has improved the quality of our diagnostic testing capabilities, which makes our overall operations more efficient and effective, but it was the smoothest system-wide technology transition that I've ever been involved with."
Converting Prime Healthcare's entire hospital system to the Masimo rainbow SET technology platform will standardize multiparameter patient monitors, oximeters, and sensors at 13 hospitals and sites of care throughout California. The oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow SET enables innovative noninvasive measurements and patient monitoring capabilities that provide real-time results for critical blood constituents and physiological parameters that help clinicians to more rapidly assess, diagnose, and treat patients.
As the only upgradable oximetry technology platform allowing hospitals to add breakthrough noninvasive blood constituent measurement capabilities that previously required invasive procedures, Masimo rainbow SET noninvasively and continuously measures total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR)—facilitating earlier detection and treatment of life-threatening conditions.
The conversion also facilitates the installation of Masimo SpHb-enabled monitors. With SpHb, clinicians have the ability to quickly measure hemoglobin levels noninvasively and continuously track them in real-time to detect low or falling hemoglobin levels that could be the result of internal bleeding. Numerous clinical studies have shown that detecting hemoglobin changes earlier with SpHb allows clinicians to intervene sooner to help protect patients from the potentially disastrous consequences of occult bleeding and post-surgical hemorrhage.1-7
Masimo Founder and CEO, Joe Kiani, stated, "Prime Healthcare is a great example of a growing trend where healthcare systems and hospital networks are leveraging advanced noninvasive medical technologies like Masimo rainbow SET to help improve patient care, outcomes, and safety."
1Ehrenfeld J., Henneman J. "Impact of Continuous and Noninvasive Hemoglobin Monitoring on Intraoperative Blood Transfusions." Massachusetts General Hospital, Harvard University Medical School. For further reading, visit: http://www.asaabstracts.com/strands/asaabstracts/searchArticle.htm;jsessionid=D1A90439AED897495AED0D43EB81C824?index=2&highlight=true&highlightcolor=0&bold=true&italic=false.
2Miller, R., Ward, T. "Changes in Noninvasive Versus Co-Oximeter Hemoglobin Values During Intraoperative Blood Loss." University of California San Francisco.
3Lamhaut, L., Apriotesei, R., Lejay, M., Vivien, B., Carli, P. "Comparison between a Technology of Spectrophotometry-Based and RBC Count for Haemoglobin Monitoring." Necker University Hospital-Assistance Publique Hopitaux de Paris, Paris, France.
4Qiu, C., LaPlace, D., Smith, M., Friedman, M., Etrata, R. "Perioperative Use of Non-Invasive Hemoglobin Monitoring: A Multi-Center Study in a Large HMO." Kaiser Permanente (Baldwin Park, San Diego, Los Angeles).
5Soliveres, J., Balaguer, J., Estruch, M., Sanchez, A., Sanchez, J. "Validation of Continuous and Noninvasive Hemoglobin Monitoring from Pulse CO-Oximetry during Surgery." University Hospital Dr Peset, Valencia, Spain.
6Richard, K., Novak, M., Dodds, T., Loftus, R., Koff, M. "Non-Invasive Measurement of Oxygen Delivery Index: Is This the Future of Goal Directed Therapy?" Dartmouth Hitchcock Medical Center, Lebanon, New Hampshire.
7Richard, K., Quill, T., Surgenor, S., Trummel, J., Koff, M. "Evaluation of Non-Invasive Hemoglobin Measurements on High Risk Surgical Patients." Dartmouth Hitchcock Medical Center, Lebanon, New Hampshire.
About Prime Healthcare Services
Prime Healthcare Services, Victorville, CA, is a progressive, innovative and rapidly expanding hospital management company in Southern California. The mission of PHS is to provide comprehensive quality healthcare in a compassionate, convenient and cost-effective manner. With more than 8,000 employees, PHS, by and through its subsidiaries, currently owns and operates twelve acute care hospitals: Centinela Hospital Medical in Inglewood (369-beds), Chino Valley Medical Center in Chino (126- beds), Desert Valley Hospital in Victorville (83-beds), Garden Grove Hospital Medical Center in Garden Grove (167-beds), Huntington Beach Hospital in Huntington Beach (131-beds), La Palma Intercommunity Hospital in La Palma (141-beds), Montclair Hospital Medical Center in Montclair (102-beds), Paradise Valley Hospital in National City (301-beds), San Dimas Community Hospital in San Dimas (93-beds), Shasta Regional Medical Center (246-beds) in Redding, Sherman Oaks Hospital in Sherman Oaks (153-beds), and West Anaheim Medical Center in Anaheim (219-beds) in California. Prime HealthCare Services Foundation owns and operates Encino Hospital Medical Center (151-beds) in Encino, California. For more information, please visit www.primehealthcare.com
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the system-wide conversion ensures that patients visiting any Prime Healthcare facility will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available, risks related to our belief that Masimo rainbow SET enables innovative noninvasive measurements and patient monitoring capabilities providing real-time results for critical blood constituents and physiological parameters that help clinicians to more rapidly assess, diagnose, and treat all patients, risks related to our belief that SpHb detects low or falling hemoglobin levels that could be the result of internal bleeding, risks related to the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Jason A. La Marca
Prime Healthcare
(909) 235-4329
jlamarca@primehealthcare.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Newly Published Study Confirms Accuracy of Masimo Noninvasive and Continuous Hemoglobin Monitor
Irvine, California – November 10, 2010 – Masimo (NASDAQ: MASI) announced today that a new study published online in the international peer-reviewed academic journal, Anesthesia & Analgesia, shows that Masimo noninvasive and continuous hemoglobin (SpHb®) monitoring technology is "accurate within 1.0 g/dL " at one standard deviation, compared to invasive blood sampling and laboratory analysis in subjects undergoing hemodilution.1 In the study, which will appear in the December 2010 print issue, researchers highlight the potential benefits of continuous noninvasive hemoglobin monitoring as "hastening the detection of postoperative bleeding, preventing the over-transfusion of blood products during surgery, reducing phlebotomy-induced anemia in the intensive care unit, and increasing patient safety and comfort in all care areas where hemoglobin testing is done."
Citing a "large potential benefit if a validated noninvasive method were available," researchers from Loma Linda University (Loma Linda, California) compared simultaneous measurements of hemoglobin using Masimo SpHb (obtained noninvasively and continuously via a Masimo Radical-7 Pulse CO-Oximeter) and laboratory CO-oximetry (tHb, obtained via an invasive blood draw of 500mL) obtained from 20 test subjects undergoing hemodilution. After initial blood draws, subjects received crystalloid IV fluid until they reached the goal of 30% reduction in hemoglobin or a max of 30mL/kg of fluid. Analyzing 355 paired measurements of noninvasive SpHb and invasive tHb, researchers found that the average difference between the two was -0.15 g/dL, 1 SD of the difference was 0.92 g/dL, and the average root-mean-square difference was 0.94 g/dL. The difference between SpHb and tHb was
Noting that an intradevice comparison of different laboratory CO-Oximeters yielded "a range of 0.1 to 1.3 g/dL difference and an average standard deviation of 0.5 g/dL," study findings indicate that Masimo SpHb measurements obtained noninvasively have clinically-acceptable accuracy and "would offer many advantages in the assessment of both acute and chronic anemic status in a variety of clinical settings."
1Macknet, M., Allard, M., Applegate, R., Rook, J. "The Accuracy of Noninvasive and Continuous Total Hemoglobin Measurement by Pulse CO-Oximetry in Human Subjects Undergoing Hemodilution" Anesth & Analg, Dec. 2010, vol 112. For further reading, visit: http://www.anesthesia-analgesia.org/content/early/2010/11/03/ANE.0b013e3181fc74b9.abstract?ct=ct.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to:risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at Lazard Capital Markets 7th Annual Healthcare Conference
IRVINE, Calif., November 5, 2010 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Lazard Capital Markets 7th Annual Healthcare Conference at The St. Regis Hotel in New York on Tuesday, November 16, at 8:55 a.m. ET. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations, Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations, Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports Third Quarter 2010 Financial Results
Product revenue grew 18% as rainbow revenue nearly doubled
Q3 2010 Highlights (compared to Q3 2009):
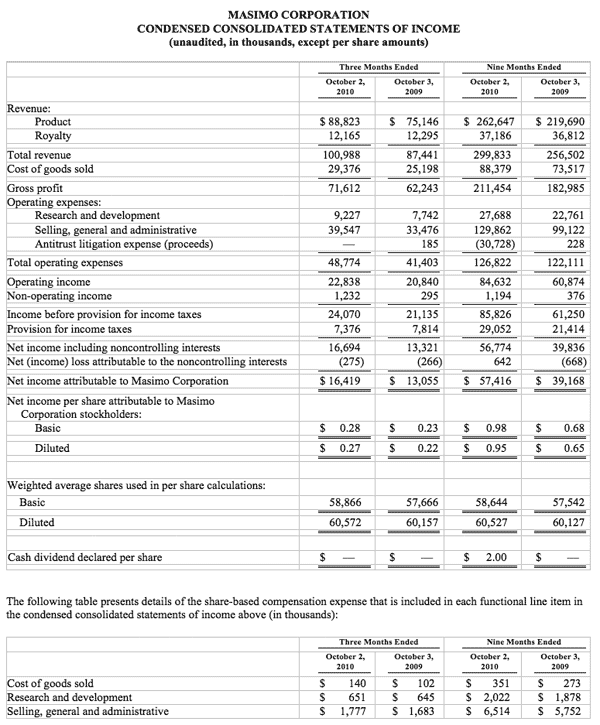
- Total revenue rose 15.5% to $101.0 million
- Product revenue rose 18.2% to $88.8 million
- Masimo SET and Masimo rainbow SET unit shipments rose 43.1% to 37,500
- Masimo rainbow revenue rose 98.7% to $11.9 million
- GAAP EPS rose 22.7% to $0.27. Excluding $0.01 in one-time expenses, adjusted EPS rose 27.3% to $0.28
Irvine, California, November 2, 2010 – Masimo Corporation (NASDAQ: MASI) today announced its financial results for the third quarter of 2010.
Masimo's total revenue for the third quarter rose 15.5% to $101.0 million, compared to $87.4 million for the third quarter of 2009. Masimo's third quarter product revenue rose 18.2% to $88.8 million, compared to $75.1 million for the third quarter of 2009. Revenue from Masimo rainbow products rose 98.7% to $11.9 million in the third quarter, compared to $6.0 million for the third quarter of 2009.
Net income for the third quarter was $16.4 million, or $0.27 per diluted share, including $0.01 per diluted share in one-time marketing-related expenses that Masimo had previously planned and announced after receiving $30.1 million in proceeds in the first quarter of 2010 from an antitrust lawsuit against Covidien. Excluding these one-time expenses, adjusted net income for the third quarter was $16.9 million, or $0.28 per diluted share, compared to net income of $13.1 million, or $0.22 per diluted share, in the third quarter of 2009.
During the third quarter, the company shipped approximately 37,500 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 43.1% compared to approximately 26,200 in the same period last year. Masimo estimates its worldwide installed base as of October 2, 2010 to be 821,000 units, up 17.8% from 697,000 units as of October 3, 2009.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Masimo continues to pursue its mission to improve patient outcomes and reduce healthcare costs by taking noninvasive monitoring to new sites and applications. Our third-quarter results demonstrate the strength of our technology and mission."
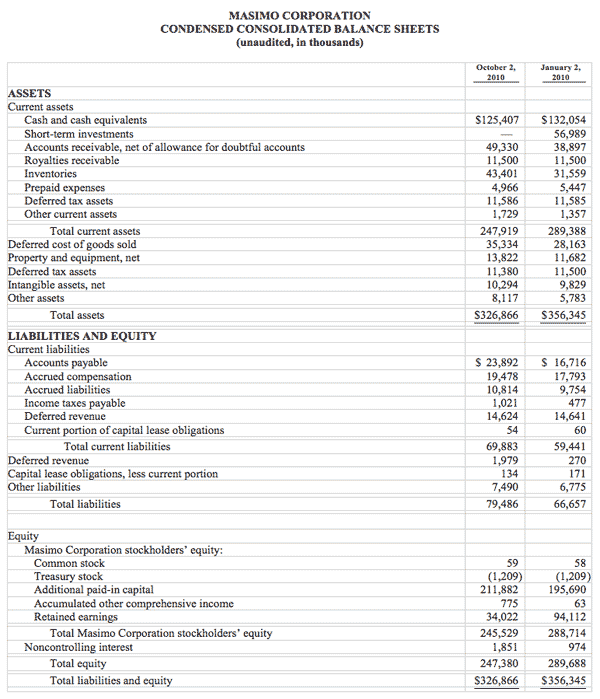
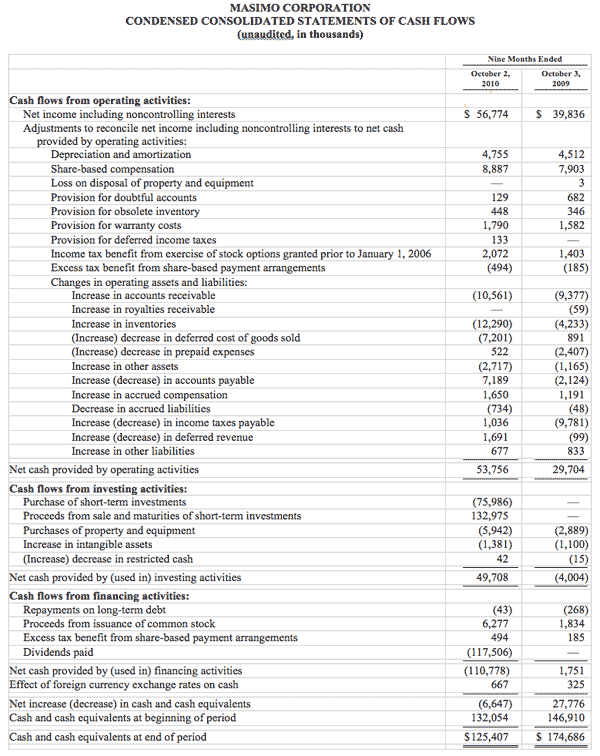
As of October 2, 2010, cash, cash equivalents and short-term investments totaled $125.4 million, compared to $189.0 million as of January 2, 2010. The decline was due primarily to the March 31, 2010 dividend payment of $117.5 million, partially offset by the net proceeds from the antitrust lawsuit and operating cash flow in the first nine months of 2010.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation code for both dial-in numbers is 17706796. After the live webcast, the call will be available on Masimo's website through December 2, 2010. In addition, a telephonic replay of the call will be available through November 16, 2010. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 17706796.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


\Over 20 New Clinical Studies Presented on Masimo Noninvasive Patient Monitoring Technologies at the American Society of Anesthesiologists Annual Meeting
Irvine, California – October 27, 2010 – Masimo (NASDAQ: MASI) announced today that over 20 new clinical studies showcasing Masimo noninvasive patient monitoring technologies were presented last week at the American Society of Anesthesiologists (ASA) Annual Meeting in San Diego, California. The largest gathering of anesthesiologists in the world, the ASA Annual Meeting provides an important platform for previewing the latest in medical developments and clinical research to industry thought-leaders.
Noninvasive and Continuous Hemoglobin (SpHb®)
This year, 10 new clinical studies examining the accuracy and clinical utility of SpHb monitoring in a variety of patient populations were conducted and presented by a variety of researchers from leading institutions around the world. Dr. Jesse Ehrenfeld, in an NIH-funded randomized controlled trial, showed SpHb monitoring reduced the frequency of intraoperative transfusions by 86% (0.6% vs. 4.5%)1 while Dr. Ronald Miller, from the University of California-San Francisco, confirmed its value in transfusion medicine, concluding that SpHb may "facilitate the decision as to whether blood should be administered"2 and Dr. Lionel Lamhaut, after evaluating patients undergoing urologic surgery using SpHb monitoring in a study conducted at Necker University Hospital in Paris, France, suggested "daily use of this technology."3
Several studies highlighted the accuracy of SpHb monitoring, including Dr. Chunyuan Qui from Kaiser Permanente who, in the first multi-center study of SpHb in a large integrated healthcare delivery network, evaluated its accuracy "over a wide range of hemoglobin values" and concluded SpHb "has the significant potential to facilitate real time decisions and to avoid the complications, expense and discomfort associated with invasive blood draws."4 Dr. Juan Soliveres, from University Hospital in Valencia, Spain, monitored surgical patients and concluded that SpHb demonstrated "clinically-acceptable accuracy and precision"5 and Dr. Kathleen Richard, at Dartmouth Hitchcock Medical Center, showed SpHb guided "early goal-directed therapy with an appropriate patient-centered algorithm in patient populations that lack more invasive access for such monitoring."6 In a separate study, Dr. Richard demonstrated SpHb's value in trending patients with significant co-morbidities during high-risk surgical procedures, noting that SpHb may "reduce serial and invasive hemoglobin measurements and tests" in the operative environment and may also "add value to care and reduce laboratory costs in many other clinical locations and scenarios (ICU, PACU, Jehovah's Witness patients, pediatrics, etc.)"7
The final three studies presented on SpHb examined its application and benefit across patient populations and procedures. At the University of Texas Medical Branch, Dr. Michael Kinsky presented his findings on two case reports demonstrating SpHb to be an "effective noninvasive indicator of vascular volume expansion" and suggested that it could "help guide fluid resuscitation during hemorrhage."8 Dr. Aubrey Yao, in a study conducted at the University of California-Davis, found SpHb to be "a feasible alternative to invasive hemoglobin monitoring" in cardiac surgery patients9 while Dr. Jochen Renner, at the University Hospital in Schleswig-Holstein, Germany, studied its accuracy in infants undergoing major surgery and concluded that SpHb showed "clinical acceptable agreement with standard laboratory hemoglobin measurement."10
Rainbow Acoustic Monitoring™
Two studies presented on Masimo's newest rainbow measurement—acoustic respiration rate (RRa™)—underscored its accuracy and applicability across hospital care areas. Dr. Jim Kumpula, at Swedish Medical Center, found RRa to be an accurate, easy, automatic method of measuring respiration rate at the bedside that could be of "significant value in a wide range of clinical settings including the general care floor, PACU, OR, sleep laboratories, and any care area utilizing conscious sedation."11 And at Loma Linda Medical Center, Dr. Mark Macknet found RAM was "better tolerated by patients and easier to use for clinical staff than capnography as some patients do not tolerate the presence of the nasal cannula needed for this technology."12
Patient SafetyNet™
Dr. Andreas Taenzer, in a study conducted on two post-surgical units at Dartmouth-Hitchcock Medical Center, found that Masimo Patient SafetyNet significantly reduced ICU transfers by 30% and rescue events in both a high-risk (vascular and cardiothoracic) and low-risk general care floor post-surgical unit.13
PVI®
Two studies demonstrated comparable accuracy between Masimo PVI for noninvasive fluid responsiveness assessments and invasive methods. Dr. Jochen Renner, at University Hospital in Schleswig_Holstein, Germany, found that PVI >14.5% "reliably predicted fluid responsiveness in infants and neonates undergoing congenital heart surgery with 80% sensitivity and specificity," but invasive CVP did not14 and Dr. Koff, at Dartmouth-Hitchcock Medical Center, showed that while Masimo PVI had "consistent agreement" with invasive methods of assessing fluid responsiveness (71%), other more manual calculation measures (PPV and SPV) were less accurate at 62%.15
Other studies presented on Masimo technologies included a study led by Dr. Soehnke Boye, at the University Medical Center in Schleswig Holstein, Germany, who "strongly recommended" Masimo SpMet monitoring for patients undergoing liposuction due to the high methemoglobinemia toxicity risks associated with use of local tumescent anesthesia, including lidocaine and prilocaine.16 Two case reports from Dr. Patrick Olomu, at the University of Texas Southwestern Medical Center at Dallas, demonstrated the "emerging utility of noninvasive technology in clinical anesthetic practice," concluding that Masimo PI is a "useful tool in assessing the efficacy of therapeutic interventions in the management of low perfusion states associated with traumatic vascular or soft tissue injury."17 And, in a study conducted at Loma Linda University, Dr. Martin Allard concluded that Masimo's brain function monitoring technology (SedLine PSA4000) "adequately and consistently reports burst suppression events as compared to the standard EEG," allowing SedLine to be used "in selected settings in place of standard EEG."18
According to Dr. Michael O'Reilly, Chief Medical Officer of Masimo, "The growing volume of independent clinical studies with Masimo technologies confirms noninvasive, continuous monitoring is taking center stage within the minds and research agendas of the world's leading clinical authorities. While it is inspiring to see such an explosion of research interest and excitement generated by our innovations, the true value of any medical technology is in what you can do clinically to benefit patients. The impact our monitoring technologies are having on both clinical practice and patient care, as evidenced by the results of the randomized control trial on SpHb, is most satisfying because it gets to the heart of our mission of 'Improving patient outcomes and reducing the cost of care by taking noninvasive monitoring to new sites and applications.'"
1 Ehrenfeld J., Henneman J. "Impact of Continuous and Noninvasive Hemoglobin Monitoring on Intraoperative Blood Transfusions." Massachusetts General Hospital, Harvard University Medical School. For further reading, visit: http://www.asaabstracts.com/strands/asaabstracts/searchArticle.htm;jsessionid=D1A90439AED897495AED0D43EB81C824?index=2&highlight=true&highlightcolor=0&bold=true&italic=false.
2 Miller, R., Ward, T. "Changes in Noninvasive Versus Co-Oximeter Hemoglobin Values During Intraoperative Blood Loss." University of California San Francisco.
3 Lamhaut, L., Apriotesei, R., Lejay, M., Vivien, B., Carli, P. "Comparison between a Technology of Spectrophotometry-Based and RBC Count for Haemoglobin Monitoring." Necker University Hospital-Assistance Publique Hopitaux de Paris, Paris, France.
4 Qiu, C., LaPlace, D., Smith, M., Friedman, M., Etrata, R. "Perioperative Use of Non-Invasive Hemoglobin Monitoring: A Multi-Center Study in a Large HMO." Kaiser Permanente (Baldwin Park, San Diego, Los Angeles).
5 Soliveres, J., Balaguer, J., Estruch, M., Sanchez, A., Snchez, J. "Validation of Continuous and Noninvasive Hemoglobin Monitoring from Pulse CO-Oximetry during Surgery." University Hospital Dr Peset, Valencia, Spain.
6 Richard, K., Novak, M., Dodds, T., Loftus, R., Koff, M. "Non-Invasive Measurement of Oxygen Delivery Index: Is This the Future of Goal Directed Therapy?" Dartmouth Hitchcock Medical Center, Lebanon, New Hampshire.
7 Richard, K., Quill, T., Surgenor, S., Trummel, J., Koff, M. "Evaluation of Non-Invasive Hemoglobin Measurements on High Risk Surgical Patients." Dartmouth Hitchcock Medical Center, Lebanon, New Hampshire.
8 Kinsky, M., Salter, M., Daneshvari, S., Indrikovs, A., George, K. "Continuous Noninvasive Hemoglobin: Impact of Hemorrhage on Volume Expansion of Crystalloid in Humans." University of Texas Medical Branch, Galveston, Texas.
9 Yao, A., Dastrange, M., Fleming, N. "Continuous Noninvasive Hemoglobin Measurement in Cardiac Surgical Patients." University of California at Davis Medical Center, Sacramento, California.
10 Renner, J., Broch, O., Scheewe, J., Gruenewald, M., Bein, B. "Non-Invasive Estimation of Hemoglobin by Pulse-CO-Oximetry in Infants Undergoing Major Surgery." University Hospital Schleswig-Holstein, Campus Kiel, Kiel, Schleswig-Holstein, Germany.
11 Kumpula, J., Harrison, R. "Accuracy of Acoustic Respiration Rate Monitoring in an Acute Nursing Unit." Swedish Medical Center, Seattle, Washington.
12 Macknet, M., Allard, M., Kimball-Jones, P., Rook, J., Applegate, R. "Accuracy of Respiratory Rate Using an Acoustic Respiration Monitor." Loma Linda University, Loma Linda, California.
13 Taenzer, A., McGrath, S., Pyke, J., Avery, J., Dodds, T. "Patient Surveillance: Transfers and Rescues on a Two Surgical Unit at Dartmouth." Dartmouth Medical School, Hanover, New Hampshire; Thayer School of Engineering, Thayer School of Engineering, Hanover, New Hampshire.
14 Renner, J., Broch, O., Scheewe, J., Gruenewald, M., Bein, B. "Non-Invasive Prediction of Fluid Responsiveness in Infants Using Pleth Variability Index." University Hospital Schleswig-Holstein, Campus Kiel, Kiel, Schleswig-Holstein, Germany.
15 Koff, M., Novak, M., Richard, K., Loftus, R., Cannesson, M. "Crossing the Threshold To Give a Fluid Bolus; Do We All Agree?" Dartmouth Hitchcock Medical Center, Lebanon, New Hampshire; Anesthesiology, University of California, Orange, California.
16 Boye, S., Opp, A., Eng., M., Klose, A., Schmeller, W., Gehring, H. "Pulse Oximeter Methemoglobin Measurements in Patients with Tumescent Anaesthesia and Prilocaine." University Medical Center Schleswig Holstein, Luebeck, Germany; Institute of Biomedical Engineering, University of Luebeck, Luebeck, Germany; Hanse-Klinik Luebeck, Luebeck, Germany.
17 Olomu, P., Romero, A., Steiner, J., Szmuk, P. "Perfusion Index – A Useful Tool To Assess Changes in Extremity Perfusion Following Major Trauma." UT Southwestern and Children's Medical Center Dallas, Dallas, Texas; Outcomes Research Consortium, The Cleveland Clinic, Cleveland, Ohio.
18 Allard, M., Macknet, M., Hsu, F., Applegate, R. "Feasibility Study: PSA Array Burst Suppression Monitoring Compared to Standard EEG in Craniotomy." Loma Linda University, Loma Linda, California; Neurosurgery, Loma Linda University, Loma Linda, California.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report Third Quarter 2010 Financial Results after Market Close on November 2, 2010
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., October 20, 2010 -- Masimo (NASDAQ: MASI) announced today that it will release third quarter 2010 financial results for the period ended October 2, 2010, after the market closes on November 2, 2010.
A conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation code for both dial-in numbers is 17706796. After the live webcast, the call will be available on Masimo's website through December 2, 2010. In addition, a telephonic replay of the call will be available through November 16, 2010. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 17706796.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Randomized Controlled Trial Shows Masimo Noninvasive and Continuous Hemoglobin Monitor Helps Clinicians Reduce Blood Transfusions During Surgery
National Institutes of Health-funded Study, Presented as Late Breaking Trial at American Society of Anesthesiologists Annual Meeting, Demonstrates 86% Reduction in Blood Transfusion Frequency
Irvine, California – October 19, 2010 – Masimo (NASDAQ: MASI) announced today that a new randomized controlled trial shows that the Masimo noninvasive and continuous hemoglobin (SpHb®) monitor helped anesthesiologists reduce the frequency of blood transfusion by 86% in patients undergoing orthopedic surgery.1 Funded by the National Institutes of Health (NIH), the study was presented as part of the late breaking trial section at the American Society of Anesthesiologists (ASA) Annual Meeting in San Diego on October 18, 2010.
In the study, researchers at Massachusetts General Hospital, Harvard Medical School, and Vanderbilt University Medical Center examined the impact of SpHb monitoring upon transfusions in a total of 327 patients undergoing orthopedic surgery during a six-month period. Patients were randomized to either a Standard Care Group (157 patients guided by invasive laboratory hemoglobin measurements alone) or Masimo SpHb Group (170 patients guided by SpHb monitoring in addition to standard care). SpHb measurements were obtained using a Masimo Radical-7® Pulse CO-Oximeter™ and rainbow® SpHb Adhesive ReSposable Sensor (R1 25). Of the 327 total surgical patients, eight received blood transfusions—seven in the Standard Care Group and only one in the SpHb Group. As a result, the frequency of intraoperative transfusions was 86% lower in the SpHb Group compared to the Standard Care Group (0.6% vs. 4.5%, p=0.03). In addition, the average number of units of blood transfused was 90% lower in the SpHb Group compared to the Standard Care Group (0.01 vs. 0.10, p=0.0001). The lack of blood transfusion in the SpHb Group did not subject those patients to added risk, as there were no differences in 30-day complication rates between groups. Researchers concluded that the "use of SpHb monitoring resulted in fewer intraoperative blood transfusions.
Additionally, researchers presented a retrospective cohort analysis, which showed that in a case-by-case matched group of 327 patients (same age, gender, and surgical procedure as each patient in the active study) taken from the six-month period prior to the study, the transfusion rate without SpHb monitoring was 4.6%. Consistent with the 4.5% transfusion rate of the Standard Care Group in the active control study, results from the cohort analysis demonstrate that SpHb monitoring made a key difference in reducing the transfusion rate.
According to lead researcher, Jesse M. Ehrenfeld, MD, MPH, recently appointed as Director, Center for Evidence Based Anesthesia at Vanderbilt University Medical Center, "Blood transfusions pose real risks to patients and are also a major contributor to the cost of surgical care. Very few monitoring technologies are subjected to the rigor of a randomized controlled trial, and even fewer are able to show a significant impact on clinician behavior and ultimate patient outcome. Our study has demonstrated that SpHb monitoring clearly changes clinician behavior and results in lower intraoperative blood transfusion rates and lower overall blood utilization. These findings have important clinical implications for hospitals around the world who are seeking to reduce surgical patient risk and reduce costs."
There is a growing consensus that blood transfusions expose patients to significantly increased morbidity and mortality risk, with evidence that each unit of blood significantly increases the risk of infection, pneumonia, sepsis, and mortality after surgery and suggests that their associated risks could be "largely avoided" through implementation of better blood management techniques and "more appropriate indicators" for transfusions.2,3 These two recent studies showed that blood transfusion increased the risk of 30-day mortality by up to 38% and morbidity by up to 40%.
The ability to noninvasively and continuously trend a patient's hemoglobin level with the Masimo rainbow technology platform offers a breakthrough in blood management and patient safety. Now, with the proven ability to reduce blood transfusions as evidenced by this study, SpHb monitoring allows clinicians to reduce patient risk and decrease blood utilization costs.
Ronald Miller, MD, MS, Anesthesiologist at University of California San Francisco and editor of "Miller's Anesthesia," stated, "These results show that SpHb monitoring enhances the decision-making process in transfusion medicine—helping to improve the basis and quality of transfusion decisions. This study helps to verify the importance of continuous SpHb monitoring with regard to blood transfusion management."
"Since we introduced SpHb in 2008, clinicians around the world have shared their success stories about how SpHb monitoring has helped them to improve the care of their patients and reduce their costs," stated Joe Kiani, Founder and CEO of Masimo. "With this new landmark study, we are happy to see Masimo SpHb become an evidence-based approach to help clinicians advance care and improve patient safety by minimizing unnecessary blood transfusions, which carry great risk and cost."
1Ehrenfeld J., Henneman J. "Impact of Continuous and Noninvasive Hemoglobin Monitoring on Intraoperative Blood Transfusions." American Society of Anesthesiologists, 2010 Annual Conference, October 18, 2010. For further reading, visit: http://www.asaabstracts.com/strands/asaabstracts/searchArticle.htm;jsessionid=5F0E35B2011BBBE191553195BFEF7C42?index=2&highlight=true&highlightcolor=0&bold=true&italic=false.
2Bernard AC et al. "Intraoperative Transfusion of 1U to 2U of Packed Red Blood Cells is Associated with Increased 30-day Mortality, Surgical Site Infection, Pneumonia, and Sepsis in General Surgery Patients." Journal of the American College of Surgeons 2009;208:931-937.
3Surgenor SD et al, for the Northern New England Cardiovascular Disease Study Group. "The Association of Perioperative Red Blood Cell Transfusions and Decreased Long-Term Survival After Cardiac Surgery." Anesthesia & Analgesia 2009; 108:1741-1746.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that using Masimo SpHb will help to reduce the frequency and quantity of blood transfusions, and risks related to our belief that Masimo SpHb provides accuracy and reliability comparable to invasive laboratory Hb measurements without its drawbacks, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Debut Halo Index, 2011 Radical-7, and New Patient SafetyNet at American Society of Anesthesiologists Meeting
Multiple New Clinical Studies Will Also Be Presented – Including a Randomized Controlled Trial Showing the Impact of Masimo SpHb Monitoring on Blood Transfusions During Surgery
Irvine, California – October 13, 2010 – Masimo (NASDAQ: MASI) announced today that several new products—including its Halo Index™, 2011 Radical-7®, and Patient Safety Net™ remote monitoring and clinician notification system—will debut in the Masimo Exhibit (#2538) at the American Society of Anesthesiologists (ASA) Annual Conference in San Diego, October 16-20, 2010. Additional highlights of the ASA Meeting include a late-breaking randomized controlled trial on the impact of Masimo noninvasive and continuous hemoglobin (SpHb®) on blood transfusions during surgery, multiple other new clinical studies, and an educational symposium on the clinical implications of Masimo's breakthrough noninvasive monitoring technologies.
Predicting Deterioration with the Halo Index
The Halo Index is a dynamic new indicator that facilitates continuous global trending and assessment of multiple physiological parameters to quantify changes in patient status. Ranging in value from 0-10, the Halo Index helps clinicians to better assess overall patient condition and predict deterioration in health status.
Redefining "Radical" with the 2011 Radical-7
When it was introduced in 2000, the Radical offered a new way to think about pulse oximetry with breakthrough measure-through motion and low perfusion capabilities and features such as SatShare™ and detachable handheld with rotational display. In 2005, the Radical-7 offered a new way of looking at patient monitoring with breakthrough rainbow® noninvasive measurement capabilities and a color display screen. Today, the 2011 Radical-7 redefines "radical" once again with more first-ever capabilities featured this week at the ASA, such as:
- rainbow®Acoustic Monitoring for accurate, easy-to-use, and patient-tolerant respiration rate
- Adaptive Threshold Alarms™ for audible notification only when significant changes in physiology have occurred
- Touch screen display for easy operation
- On-the-fly configuration of parameter size and waveforms
- MyView™ for personalized displays through presence detection
- Integrated wireless 802.11 and Bluetooth for seamless connectivity
Defining General Floor Monitoring with Masimo rainbow SET & Patient SafetyNet
The Patient SafetyNet system is the only general floor monitoring system proven to help clinicians reduce rescue activations, ICU transfers, and ICU days.1 With new expanded capabilities debuting at the ASA, Patient SafetyNet takes enhanced and automated care to a new level with:
- Halo Index for global trending and assessment of physiological parameter trends
- Real-time or playback of breath sounds to assist clinicians in respiratory assessment
- Plethysmographic and acoustic waveforms for real-time or playback patient assessment
- MyView for personalized displays through presence detection technology
- Clinician workflow analytics to help improve efficiency
Multiple Clinical Studies Highlight Impact of Masimo Technologies
This week's ASA meeting will also include 20 new clinical studies on Masimo technologies. Eleven studies demonstrate the accuracy and clinical utility of SpHb monitoring, including a randomized controlled trial conducted at Massachusetts General Hospital.
Non-CME Educational Symposium Showcases Clinical Implications of Masimo's Breakthrough Solutions
On Monday, October 18, 2010, 6:30-8:30 p.m., four anesthesiology thought-leaders will present critical insights on the clinical implications of Masimo's breakthrough solutions, including SpHb, PVI, rainbow acoustic monitoring, and Patient SafetyNet.
The symposium will feature the following presentations and speakers:
Improving Outcomes on the General Floor with Measure-Through Motion Pulse Oximetry and Remote Monitoring and Notification
George Blike, MD—Medical Director, Patient Safety and Training Center, Dartmouth-Hitchcock Medical Center.
Goal-Directed Fluid Management with Noninvasive and Continuous Pleth Variability Index
Maxime Cannesson, MD, PhD—Associate Professor of Anesthesiology, University of California-Irvine.
Noninvasive & Continuous Hemoglobin: Clinical Application and Impact on Surgical Blood Management
Ronald Miller, MD—former Chairman, Department of Anesthesiology, University of California-San Francisco, and Editor of "Miller's Anesthesia", the definitive textbook for anesthesiology.
Accurate and Patient-Tolerant Respiration Rate with Acoustic Monitoring
Michael Ramsay, MD—Chief of Service, Department of Anesthesiology & Pain Management, Baylor University Medical Center-Dallas, and President, Baylor Research Institute.
*Some of the new product features and enhancements described have not been FDA 510(k) cleared.
1 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading, visit: http://journals.lww.com/anesthesiology/Fulltext/2010/02000/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.10.aspx.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the features, performance, expanded capabilities, and commercial availability of Masimo's 2011 product technologies, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


New Multi-Center Study Finds Clinical Practice Change with Masimo SET Pulse Oximetry Reduces Severe Eye Damage More Than 50% in Premature Newborns
Only Use of Masimo SET Reduced Severe Blindness and Corrective Laser Surgery
Irvine, California – September 30, 2010 – Masimo (NASDAQ: MASI) announced today that a new multi-center study published in the International Peer-Reviewed Academic Journal, Acta Paediatrica, shows that a change in clinical practice with the use of Masimo SET pulse oximetry technology led to a significant reduction of severe Retinopathy of Prematurity (ROP)—a devastating eye disease resulting in partial or complete blindness—in premature newborns. The study also confirmed that conventional pulse oximetry technology is not effective in reducing ROP, even when clinical practice is changed to reflect lowered oxygen saturation targets.1
ROP is the second leading cause of blindness in childhood in the United States—affecting over 20% of premature babies. A major cause of ROP is the use of excess oxygen to treat respiratory problems in premature babies,2 which stimulates abnormal vessel growth within the eye. Although the use of pulse oximetry is established as a standard-of-care technology for measuring oxygen saturation and appropriately titrating oxygen administration to prevent ROP, accuracy and reliability varies greatly by which pulse oximetry technology is used. Masimo SET pulse oximetry technology is clinically-proven to measure-through motion and low perfusion, leading to accurate and reliable monitoring of oxygen saturation in premature newborns. Although previous clinical studies have shown a reduction in the incidence of ROP in premature newborns when Masimo SET pulse oximetry is used in combination with appropriate titration of oxygen administration,3,4 this is the first published study showing a head-to-head comparison of Masimo SET vs. another "next generation" pulse oximetry.
In the current study, researchers examined the role that SpO2 technology plays in the prevention of ROP in 571 high-risk premature newborns (weighing less than 1,250 grams / 2.75 lbs) at two Emory University (Atlanta, GA) hospitals during two consecutive three-year periods (Periods 1 and 2) and an 18-month follow-up period (Period 3). During Period 1, Nellcor pulse oximeters were used in both centers and oxygen saturation targets were set at 92-100%. In Period 2, new practice guidelines for lower oxygen saturation targets of 88-93% for premature infants were implemented at both centers, but only one center switched to Masimo SET oximeters. In Period 3, the center previously using Nellcor oximeters switched to Masimo SET.
Study results showed that in Period 1, when both centers were using Nellcor, ROP rates were not different between centers. In period 2, when both centers implemented a practice change, the center switching to Masimo SET reduced ROP rates from 12% to 5% while the center using Nellcor did not reduce ROP rates at all, remaining at a 13% ROP rate. In period 3, the center previously using conventional pulse oximetry switched to Masimo SET and reduced ROP rates from 13% to 6%. Researchers concluded that the "reduction in the incidence of severe ROP and need for laser therapy was associated with the use of signal extraction pulse oximetry (Masimo SET)" and the study "findings lend further support to the significance of using improved saturation monitors in managing critically-ill infants."
According to lead researcher, Armando Castillo, MD, Neonatologist at Northeast Georgia Medical Center, "Our study findings show that Masimo SET is not only clinically beneficial, but uniquely impactful for reducing the incidence and prevalence of ROP in Extremely Low Birth Weight infants. As such, NICU clinicians wanting to use evidence-based strategies should seriously consider implementing Masimo SET."
1Castillo A., Deulofeut R., Critz A., Sola A. "Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology." Acta Paediatrica, accepted article published online ahead of print http://onlinelibrary.wiley.com/doi/10.1111/j.1651-2227.2010.02001.x/abstract.
2Naveed Hussain, Jonathan Clive, and Vineet Bhandari. "Current Incidence of Retinopathy of Prematurity." 1989-1997 Pediatrics, Sep 1999; 104: e26.
3Chow L, Wright K, Sola A. "Can Changes in Clinical Practice Decrease the Incidence of Severe Retinopathy of Prematurity in Very Low Birth Weight Infants?" Pediatrics 2003; 111(2):339-345.
4Sola A., Rogido, Marta, Deulofeut, Richard. "Oxygen as a Neonatal Health Hazard: Call for Dtente in Clinical Practice." Acta Paediatrica 2007; 96:801-812.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that using Masimo SET pulse oximetry technology will help to dramatically reduce the incidence of ROP, risks related to our belief that Masimo SET provides superior sensitivity and specificity to more accurately and reliably measure oxygen saturation in premature newborns weighing less than 1,250 grams than conventional pulse oximetry technologies, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
phone: (949) 297-7348
email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.

Two Clinical Case Reports Demonstrate Trend Accuracy of Masimo Noninvasive and Continuous Hemoglobin and Illustrates Its Use as an Effective Tool for Blood Conservation
Irvine, California – September 28, 2010 – Masimo (NASDAQ: MASI) announced today that two new clinical case reports presented at the Society for the Advancement of Blood Management (SABM) Annual Meeting demonstrate that Masimo noninvasive and continuous hemoglobin (SpHb®) provides measurements as accurate as invasive laboratory blood tests, offering a "new and effective strategy to facilitate blood conservation efforts and minimize unnecessary transfusions in the intensive care unit (ICU)." The case report also states that SpHb monitoring contains none of the drawbacks of traditional blood analysis that can impact clinical decisions.
According to Aryeh Shander, MD, Executive Medical Director at the Institute for Patient Blood Management & Bloodless Medicine Surgery and Chief of Anesthesiology and Critical Care Medicine at Englewood Hospital & Medical Center, "In continuing to recognize the negative impact of red blood cell (RBC) transfusion on the critically-ill, our threshold for transfusion is increasing. Masimo noninvasive and continuous hemoglobin technology offers two significant advantages: the first is the ability to continuously monitor hemoglobin (especially at low levels) as we treat the patient with modalities other than transfusion and the second is the ability to avoid or reduce development of anemia in the ICU patients due to repeated blood draws that cause and aggravate anemia as noninvasive hemoglobin measurement eliminates the need for repeated blood draws in anemic patients."
In the first case report presented, researchers at the Englewood Hospital and Medical Center (EHMC) in New Jersey and Mt. Sinai School of Medicine in New York tracked the performance of Masimo SpHb in a severely anemic patient over the course of four days in the ICU. In comparing SpHb with invasive hemoglobin measurements (tHb), researchers found high correlation with a SpHb bias of 1.26 g/dL and standard deviation of 0.43 g/dL. Researchers noted that SpHb measurements (ranging from 4.9 to 11.2 g/dL) "exhibited a consistent trend compared to the changes in laboratory hemoglobin measurements" (between 5.2 to 8.1g/dL) over the four-day period, concluding that "the ability to continuously monitor hemoglobin noninvasively may offer a new and effective strategy to facilitate blood conservation efforts and minimize unnecessary blood transfusions in the ICU."1
In the second case report presented, researchers at Mayo Clinic in Jacksonville, Florida tracked the performance of Masimo SpHb in a patient undergoing high blood loss liver transplantation surgery. Comparing SpHb with invasive hemoglobin measurements (tHb) led researchers to conclude that Masimo SpHb measurements compared "favorably with the laboratory measured values over a range of 6-12 g/dL." Researchers also noted the unique advantages of SpHb monitoring in that it provides "results faster and as an instantaneous readout" while the "stored data is easily retrievable enabling post-procedure processing." Dr. Klaus Torp, lead researcher and Anesthesiologist at Mayo Clinic, recognizes the importance and impact of SpHb monitoring in surgical patients, offering that "During massive hemorrhage, having the ability to monitor hemoglobin levels in real-time may enable the anesthesiologist to assess and match transfusion needs fast—allowing one to rapidly optimize oxygen delivery, intravascular volume, and avoid over-transfusion."2
Traditional blood analysis has many drawbacks, including complexity, time-consuming turnaround times that can impact clinical decisions, and blood loss due to invasive blood draws that have been found to contribute substantially to the anemic conditions that commonly occur in critically-ill patients—thereby increasing transfusion rates. Published studies have shown that transfusion of just one or two units of blood significantly increases infection, pneumonia, sepsis, and mortality after surgery.3,4 These studies also suggest that transfusions and their associated risks could be "largely avoided" through implementation of better blood management techniques and "more appropriate indicators" for transfusions. The ability to continuously and noninvasively trend a patient's hemoglobin level with Masimo SpHb offers a breakthrough in blood management with the potential to improve clinical decision-making, reduce patient exposure to unnecessary blood transfusions, and preserve precious resources.
"These two case reports clearly demonstrate that Masimo SpHb provides the best available opportunity for reducing and/or eliminating anemia and transfusion related risks in surgical patients in the easiest and quickest possible way," stated Dr. Shander.
SpHb is available as part of the Masimo rainbow® SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR).
1 Juhl A., Naqvi S., Aregbeyen O., Shander A. "Noninvasive and Continuous Hemoglobin Monitoring in an Intensive Care Unit Patient with low Hemoglobin." Society for the Advancement of Blood Management (SABM) Annual Meeting. September 24, 2010.
2 Prith P, Torp K, et al. Real Time Hemoglobin Monitoring During Liver Transplantation. A Case Report. Society for the Advancement of Blood Management (SABM) Annual Meeting. September 24, 2010.
3 Bernard AC et al. Intraoperative transfusion of 1U to 2U of packed red blood cells is associated with increased 30-day mortality, surgical site infection, pneumonia, and sepsis in general surgery patients. Journal of the American College of Surgeons. 2009;208:931-937.
4 Surgenor SD et al, for the Northern New England Cardiovascular Disease Study Group. The Association of Perioperative Red Blood Cell Transfusions and Decreased Long-Term Survival After Cardiac Surgery. Anesthesia & Analgesia 2009; 108:1741-1746.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpHb will facilitate early diagnosis and treatment of low hemoglobin/anemic conditions for all surgical and post-surgical patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


New Clinical Study Finds Masimo Noninvasive SpMet is Accurate Compared to Invasive Methemoglobin Testing
Study Published in Anesthesia & Analgesia Indicates SpMet Offers Advantages Including Immediate Detection and Less Time and Effort Than Traditional Methods
Irvine, California – September 21, 2010 – Masimo (NASDAQ: MASI) announced today that a new clinical study published in Anesthesia & Analgesia demonstrates that Masimo noninvasive and continuous methemoglobin (SpMet®) measurements provide a high degree of agreement in measurements with corresponding laboratory values of methemoglobin obtained by invasive methemoglobin testing In addition, the authors concluded that SpMet could facilitate early diagnosis and treatment of methemoglobinemia during high-risk procedures.1
Methemoglobin (metHb) is an oxidized form of hemoglobin that is incapable of oxygen transport. Methemoglobinemia is defined as higher than normal methemoglobin levels and can be induced by common topical anesthestics (e.g. Benzocaine, Cetacaine, Prilocaine, Lidocaine) and over 30 other therapeutic drugs prevalent in both the hospital and outpatient settings. If undetected and untreated, methemoglobinemia can result in significant reduction in oxygen delivery to the tissues resulting in vital organ damage.2 Prevention of vital organ injury requires prompt detection and treatment as significant organ injury can occur in as little as five minutes. However, traditional methods of detection rely on physical signs and symptoms, which may be non-specific and subtle or invasive laboratory blood tests that that can take a long time. Masimo SpMet provides an accurate, quick, and easy-to-use way to noninvasively and continuously measure methemoglobin levels in the blood, which helps clinicians assess methemoglobinemia to determine treatment and additional test options.
In the current study, researchers from the University Clinic of Schleswig-Hostein in Luebeck, Germany, compared the accuracy of SpMet from the Masimo Radical-7 Pulse CO-Oximeter with invasive arterial blood gas measurements in 40 patients receiving prilocaine as a regional anesthetic for orthopedic surgery. A total of 20 patients received an interscalene brachial plexus block with 300 mg prilocaine while the other 20 patients received a combined femoral-sciatic nerve block with two 300 mg injections of prilocaine (600 mg total). Continuous monitoring of SpMet and SpO2 was initiated before the onset of regional anesthesia and blood gas analysis of methhemoglobin and oxygen saturation (SaO2) was performed at the first injection of prilocaine and at 15, 30, 60, 120, 180, 240, 300, and 360 minutes. Study results showed a "high degree of agreement in measurement" between noninvasive and continuous SpMet and SpO2 and corresponding laboratory values of methemoglobin and SaO2 with a bias of 0.27% and 95% confidence limits of +1.33%—leading researchers to conclude that Masimo SpMet "may facilitate early diagnosis and treatment, when necessary, of dyshemoglobinemia."
According to Steven J Barker, PhD, MD, Professor and Head of the Department of Anesthesiology at University of Arizona and Masimo Board Member, "I've seen the lifesaving benefits of Masimo SpMet monitoring first-hand when a surgical patient developed potentially fatal methemoglobin levels from local anesthestic toxicity. Traditional laboratory blood testing did not provide results quickly enough to diagnose and treat this critically-ill patient. Thankfully, SpMet provided immediate detection of her methemoglobinemia and successfully guided our treatment."
SpMet is available as part of the Masimo rainbow® SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR).
"Unrecognized methemoglobinemia is a patient safety problem in every hospital on the planet; however, most healthcare providers are not aware of the number of drugs that cause methemoglobinemia, which is not uncommon and often goes unrecognized," stated Michael O'Reilly, MD, EVP of Medical Affairs at Masimo "Over 30 drugs have been shown to induce severe methemoglobinemia, including commonly-used local anesthesics such as Lidocaine, Benzocaine, and Prilocaine; antibiotics like Trimethoprim, Sulphonamides, and Dapsone; and many other therapeutic drugs such as Metoclopramide, Chlorates, Nitrates and Bromates to name a few. The morbidity risks associated with methemoglobin, which significantly decreases oxygen delivery, is completely underappreciated and neonates and infants less than 6 months of age are particularly susceptible because of their immature metabolic pathways. Given its prevalence and susceptible populations, I believe that SpMet offers a critical opportunity to noninvasively and continuously monitor every patient for this very serious, unrecognized and potentially deadly problem."
1Soeding, Peter, Deppe, Matthias, Gehring, Hartmut. "Pulse-Oximetric Measurement of Prilocaine-Induced Methemoglobinemia in Regional Anesthesia." Anesthesia & Analgesia. August 2010. Published online http://www.anesthesia-analgesia.org/content/early/2010/08/12/ANE.0b013e3181eb6239.abstract?ct=ct.
2Ash-Bernal R, Wise R, Wright SM. "Acquired Methemoglobinemia: A Retrospective Series of 138 Cases at 2 Teaching Hospitals." Medicine 2004; 83:265-272. For further reading, visit: http://journals.lww.com/md-journal/pages/articleviewer.aspx?year=2004&issue=09000&article=00001&type=abstractre.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpMet will facilitate early diagnosis and treatment of methemoglobinemia for all surgical patients receiving local anesthesia, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Noninvasive and Continuous Hemoglobin Receives Market Clearance in Japan
Irvine, California – September 15, 2010 - Masimo (NASDAQ: MASI) announced today the Japanese Ministry of Health, Labor and Welfare (MHLW) regulatory clearance of its noninvasive and continuous hemoglobin (SpHb) measurement, available as part of the Masimo rainbow SET® platform. Representing a breakthrough solution for noninvasively and continuously measuring a patient's hemoglobin level in real-time, SpHb tracks and trends hemoglobin levels without needles, time-consuming laboratory analysis, and risk of contamination associated with traditional blood tests.
The availability of noninvasive, continuous, and immediate hemoglobin measurements is expected to have wide-ranging clinical impact in Japan—from surgery and intensive care units to less acute care settings, including the emergency department and physician offices. SpHb may help clinicians determine whether a patient has a low hemoglobin level (known as anemia) and facilitate prompt detection of internal bleeding and more appropriate administration of blood transfusions—by referring to the SpHb measurement that is continuously displayed on Masimo rainbow SET Pulse CO-Oximeters™.
Now, clinicians in Japan can benefit from the full range of physiologic measurements available on the Masimo rainbow SET platform—including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR).
Joe Kiani, Masimo Founder and CEO, stated, "Receiving full MHLW regulatory approvals for SpHb makes the noninvasive, continuous measurement of hemoglobin universally available to Japanese clinicians. This arms clinicians throughout Japan with the real-time assessment of hemoglobin—enabling more appropriate management of patients both inside and outside of the operating room."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpHb will facilitate real-time hemoglobin measurements for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Palmetto Health Richland Hospital Becomes First in South Carolina to Noninvasively, Wirelessly Monitor Patients Using State-of-the-Art Masimo Patient SafetyNet™ System
System Installed to Help Clinicians Improve Safety, Speed Recoveries, Increase Efficiencies, and Reduce the Cost of Care at Palmetto Children's Hospital and Heart Hospital
Irvine, California – September 14, 2010 – Palmetto Health Richland in Columbia—part of Palmetto Health, South Carolina's largest and most comprehensive healthcare resource, and Masimo (NASDAQ: MASI) today jointly announced South Carolina's first installation of Masimo Patient SafetyNet, an advanced remote monitoring and wireless clinician notification system designed to facilitate appropriate early clinical response, preempt sentinel events, and avoid unnecessary intensive care unit (ICU) transfers while helping hospitals to meet Joint Commission, Anesthesia Patient Safety Foundation (APSF), and American Society of Anesthesiologists (ASA) clinical practice guidelines for patient safety.
Preventable patient deaths and injuries associated with failure to rescue events are one of today's most common medical errors.1 But today, hospitals have a new tool helping them to prevent failure to rescue events and improve patient safety. The Patient SafetyNet system allows clinicians at Palmetto Health-Richland to noninvasively, continuously, and remotely monitor multiple physiological measurements—including arterial oxygen saturation, perfusion index, and pulse rate—for up to 80 patients simultaneously from a central location, facilitating early detection of physiological abnormalities that prompts earlier intervention. In a recently-published landmark study, Patient SafetyNet was shown to help decrease distress codes and rescue activations by 65% and intensive care unit (ICU) transfers by 48%—saving 135 ICU days annually.2
According to Leah Inman, RT, Director of Respiratory Care at Palmetto Health Richland, "We are always looking to improve the quality of care for our patients and the Patient SafetyNet system is another way for us to do this."
When a patient is in distress, specific, detailed physiological data is transmitted to assigned clinicians, enabling them to intervene before the situation becomes critical and requires more acute levels of care. This is particularly important for post-surgical patients who are at increased risk of injury or death resulting from the respiratory depression effects of medications used for sedation and pain management.
Masimo Founder and CEO, Joe Kiani, stated, "Palmetto Health is a shining example of caring high-tech healthcare. With a long-standing tradition of deploying the latest technology and treatment protocols for quality patient care, they have been recognized as one of the '99 Most Wired Hospitals' and rewarded with the highest level of patient satisfaction in the nation. Their recent implementation of Patient SafetyNet adds another layer of patient safety and dimension of care in their efforts to improve the human condition."
1 "The Sixth Annual HealthGrades Patient Safety in American Hospitals Study" April 2009. For further reading, visit: http://www.healthgrades.com/media/DMS/pdf/PatientSafetyInAmericanHospitalsStudy2009.pdf.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading, visit: http://journals.lww.com/anesthesiology/Fulltext/2010/02000/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.10.aspx.
About Palmetto Health
Palmetto Health is the region's largest, most comprehensive, locally owned, not-for-profit health care resource. The 1,138-bed system in Columbia, South Carolina, is composed of four outstanding hospitals: Palmetto Health Richland and Baptist in Columbia, and the Heart Hospital and Children's Hospital in Columbia. Palmetto Health leads the region in the number and volume of inpatient and outpatient services provided—providing care for 70 percent of the residents of Richland County and more than 55 percent of the healthcare for the combined Richland/Lexington county area. Palmetto Health Children's Hospital—South Carolina's first free-standing children's hospital— treats more than 80,000 children each year. It has central South Carolina's only Children's Emergency Center and offers more than 30 subspecialties to meet the unique healthcare needs of children. Palmetto Health Heart Hospital is South Carolina's only freestanding hospital dedicated solely to the prevention, diagnosis and treatment of cardiovascular disease with 124 all-private inpatient beds. Utilizing superior technology to perform high-risk surgeries, Palmetto Health Heart Hospital's commitment to quality care and unmatched quality measures results in consistent outcomes—including low overall complication and mortality rates and the highest patient satisfaction ratings in the nation. Visit www.palmettohealth.org for more information.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Patient SafetyNet will provide an effective early warning system of a patient's deteriorating physiological condition in real-time for all patients to enable timely rescue and increase patient safety, risks related to our belief that Masimo SET and Masimo Rainbow SET measurements and alarms will provide sufficient sensitivity and specificity to detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Tammie Epps
Palmetto Health Richland Hospital
(803) 434-4903
tammie.epps@palmettohealth.org
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at Stifel Nicolaus 2010 Healthcare Conference
IRVINE, Calif., September 8, 2010 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Stifel Nicolaus 2010 Healthcare Conference at the Four Seasons Hotel in Boston on Wednesday, September 15, at 8:00 a.m. ET. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Banner Health Converts 23-Hospital System to Masimo rainbow SET Technology
One of the Largest Nonprofit Health Systems in the U.S. Standardizes Care to Masimo SET and Adds 200 Noninvasive Hemoglobin Monitoring Devices and Two Patient SafetyNet Systems within its Network
Irvine, California – September 2, 2010 – Banner Health and Masimo (NASDAQ: MASI) today jointly announce Banner Health's system-wide conversion to Masimo rainbow® SET technology. The system-wide conversion ensures that patients visiting any Banner Health hospital will be cared for using the most technologically and clinically-advanced oximetry and noninvasive patient monitoring solutions available.
"The decision to convert our entire hospital system to Masimo technology was driven by a simple initiative to ensure that every Banner Health hospital provides leading-edge medical care," stated John Hensing, Executive Vice President and Chief Medical Officer at Banner Health. "Masimo's rainbow SET platform and patient monitoring solutions offer the most advanced noninvasive measurement capabilities available, providing unique patient care advantages and cost savings."
Masimo Founder and CEO, Joe Kiani, stated, "The significance of a health system of this size and status standardizing on Masimo rainbow SET technology system-wide, placing over 200 noninvasive hemoglobin monitors throughout their hospital network, and installing multiple Patient SafetyNet systems is huge in terms of demonstrating a care provider's commitment to advancing the practice of healthcare and patient safety. Banner Health's adoption of our advanced noninvasive technologies shows that Masimo rainbow SET, noninvasive hemoglobin monitoring, and Patient SafetyNet are key drivers of medical advancements for hospitals worldwide."
Converting Banner Health's entire hospital system to Masimo technology will standardize multiparameter patient monitors, oximeters, and sensors at 23 hospitals and sites of care in seven states. The oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow SET enables innovative noninvasive measurements and patient monitoring capabilities that provide real-time results for critical blood constituents and physiological parameters that help clinicians to more rapidly assess, diagnose, and treat patients.
As the only upgradable oximetry technology platform allowing hospitals to add breakthrough noninvasive blood constituent measurement capabilities that previously required invasive procedures, Masimo rainbow SET continuously and noninvasively measures total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to Masimo SET 'gold standard' Measure-Through Motion and Low Perfusion oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR) measurements enables monitoring of multiple physiological parameters simultaneously—facilitating earlier detection and treatment of life-threatening conditions.
The conversion will also facilitate the installation of 200 Masimo SpHb-enabled monitors and two Masimo Patient SafetyNet remote monitoring and wireless clinician notification systems. With SpHb, clinicians have the ability to quickly measure hemoglobin levels noninvasively and continuously track them in real-time to detect low or falling hemoglobin levels that could be the result of internal bleeding. Detecting hemoglobin changes earlier allows clinicians to intervene sooner to help protect patients from the potentially disastrous consequences of occult bleeding and post-surgical hemorrhage.
Masimo SpHb and PVI have been shown in numerous clinical studies to provide accurate, reliable, real-time measurements that may help clinicians to more tightly monitor and proactively manage hemoglobin and fluid levels. And, with the Patient SafetyNet system, clinicians can keep patients safer by continuously, noninvasively, and remotely monitoring multiple physiological parameters, and automatically alerting clinicians to changes that signal patient distress or deterioration. Clinically-proven to reduce rescue events and activations by 65% and ICU transfers by 48%, Patient SafetyNet contributes to significant improvements in patient outcomes.1
1 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading, visit: http://journals.lww.com/anesthesiology/Fulltext/2010/02000/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.10.aspx
About Banner Health
Headquartered in Phoenix, Banner Health is one of the largest, nonprofit health care systems in the country. The system owns or leases 23 acute-care hospitals, long-term care centers, outpatient surgery centers and an array of other services including family clinics, home care and hospice services, and a nursing registry. Banner Health is in seven states: Alaska, Arizona, California, Colorado, Nebraska, Nevada and Wyoming.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the system-wide conversion ensures that patients visiting any Banner Health facility will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available, risks related to our belief that Masimo rainbow enables innovative noninvasive measurements and patient monitoring capabilities providing real-time results for critical blood constituents and physiological parameters that help clinicians to more rapidly assess, diagnose, and treat all patients, risks related to our belief that SpHb detects low or falling hemoglobin levels that could be the result of internal bleeding, risks related to the repeatability of clinical results, and our assumptions that the Patient SafetyNet system will allow clinicians to keep patients safer by continuously, noninvasively, and remotely monitoring multiple physiological parameters, and automatically alerting them to changes that signal patient distress or deterioration, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Bill Byron
Banner Health
(602) 747-4000
bill.byron@bannerhealth.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Randomized Controlled Trial Finds Masimo PVI Improves Fluid Management During Surgery
Study Published in Anesthesia & Analgesia Shows PVI-Guided Therapy Reduced Fluid Administered and Led to Lower Lactate Levels
Irvine, California – August 31, 2010 – Masimo (NASDAQ: MASI) announced today that a new randomized controlled trial published in Anesthesia & Analgesia shows that clinicians using Masimo Pleth Variability Index (PVI®) significantly improved fluid management and reduced lactate levels in patients during and after surgery, compared to patients managed by standard care without PVI1. Multiple previous studies2,3,4 have shown that PVI predicts fluid responsiveness, defined as a significant increase in cardiac output after fluid administration, but this is the first published study to show that the use of PVI can improve patient management compared to a group of patients not managed with PVI.
Although fluid administration is critical to optimizing patient status and enabling end organ preservation, unnecessary fluid administration is associated with increased morbidity and mortality. Traditional invasive measurements such as central venous pressure (CVP) are not reliable in predicting whether a patient will benefit from fluid administration, and newer methods of predicting fluid responsiveness are invasive and/or costly. Masimo PVI provides clinicians with a noninvasive, continuous, and cost-effective measure in assessing whether patients will benefit from fluid administration to enable more personalized and targeted fluid therapy.
In the current study, researchers from the University Catholique de Louvain, St. Luc Hospital in Brussels, Belgium, randomized 82 patients undergoing abdominal surgery into two groups, a control group where fluid management was guided by standard care through CVP and clinician assessment, and the PVI group where fluid management was guided by standard care and PVI values from a Masimo Radical-7® Pulse CO-Oximeter®. In the PVI group, 500mL of crystalloids were infused at induction, followed by 2mL per kg per hour continuous infusion. A 250mL bolus of colloid was added if the PVI exceeded 13% for more than 5 minutes. In the control group, 500mL of crystalloids were infused at induction, followed by continuous infusion of crystalloids (4 to 8mL per kg per hour) and a 250mL bolus of colloids was given to compensate acute blood losses (>50mL), maintain mean arterial pressure above 65mmHg and the central venous pressure above 6mmHg.
The results showed that the PVI group received significantly lower amounts of intraoperative crystalloids (P=0.004) and total volume infused (P=0.049), and lactate levels were significantly lower during surgery (1.2 mmol/L +/- 0.6 vs 1.6 +/- 1.2, P=0.04), 24-hours post-operatively (1.4 +/- 0.3 vs 1.8 +/- 1.0, P=0.02), and 48-hour post-operatively (1.2 +/- 0.3 vs 1.4 +/- 0.4, P=0.03). The researchers concluded that "PVI-based goal directed fluid management reduced the volume of intraoperative fluid infused and reduced both intraoperative and postoperative lactate levels." Study authors also noted that the reduction in lactate levels for PVI-guided patients suggests that "PVI-guided fluid management may lead to fluid administration that is tailored to each individual patient's needs."
PVI is available as part of Masimo rainbow® SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), perfusion index (PI), and acoustic respiration rate (RRa™), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
"The clinical merit of using a dynamic index like PVI to guide fluid administration relates to a timing issue," stated William E. Johnston, MD, Professor and Associate Chair, Vice Chair of Academic Affairs in the Department of Anesthesiology at Scott & White Memorial Hospital in Temple, Texas. "What's so unique about PVI is that it allows the medical team to rapidly fine tune fluid administration using a Masimo Radical-7 Pulse CO-Oximeter in the operating room before global hypovolemia and hypoperfusion occur. Consequently, an appropriate amount of fluid can be administered at the most opportune time."
1 Forget, Patrice; Lois, Fernande; De Kock, Marc."Goal-Directed Fluid Management Based on the Pulse Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management." Anesthesia & Analgesia. August 2010. Published online ahead of print http://www.anesthesia-analgesia.org/content/early/2010/08/12/ANE.0b013e3181eb624f.abstract?ct=ct.
2 Cannesson M, Desebbe O, Rosamel P, Delannoy B, Robin J, Bastien O, Lehot JJ. "Pleth variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre." British Journal of Anaesthesia August 2008; 101(2):200-6. For further reading, visit: http://www.ncbi.nlm.nih.gov/pubmed/18522935.
3 Markus Zimmerman, Thomas Feibicke, Cornelius Keyl, Christopher Prasser, Stefan Moritz, Bernhard M. Graf, and Christoph Wiesenack. "Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically-ventilated Patients Undergoing Major Surgery." European Journal of Anaesthesiology June 2010; 27(6):555-61. For further reading, visit: http://www.ncbi.nlm.nih.gov/pubmed/20035228.
M. Feissel, R. Kalakhy, J. Badie, G. Robles, J. Faller, JL. Teboul. "Plethysmography Variability Index: A New Fluid Responsiveness Parameter." Presented at the 29th International Symposium on Intensive Care and Emergency Medicine (ISICEM) Annual Meeting, March 25, 2009,Brussels, Belgium. For further reading, visit: http://ccforum.com/content/13/S1/P205.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo PVI will help to improve perioperative fluid management for all surgical patients, and risks related to our assumptions that Masimo PVI-guided fluid management during surgery will help to reduce hypotension and improve circulatory status and renal function in all surgical patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Frankfurt am Main Emergency Medical Services Makes Masimo Rad-57 Pulse CO-Oximeters Mandatory for All Vehicles
One of Largest Cities in Germany Mandates Carbon Monoxide Screening with Masimo rainbow SpCO®
Irvine, California – August 26, 2010 – Frankfurt am Main Emergency Medical Services (EMS) in Germany and Masimo (NASDAQ: MASI), the inventor of rainbow® Pulse CO-Oximetry™, rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, today jointly announced that every EMS vehicle in the city of Frankfurt, Germany will be equipped with a Masimo Rad-57® Pulse CO-Oximeter—making carbon monoxide (CO) screening by Pulse CO-Oximetry a city-wide EMS requirement.
Frankfurt am Main is the first city in Germany to mandate the use of Masimo Rad-57 for CO screening and to equip every ambulance in town with this lifesaving technology. More than 30 Rad-57 Pulse CO-Oximeters, which noninvasively measure the amount of CO in the bloodstream, were purchased to protect the health and safety of Frankfurt citizens and paramedics from the dangers of undetected CO poisoning.
Carbon monoxide—an odorless, colorless, toxic gas that is impossible to see, taste or smell—is the leading cause of injury and death by poisoning worldwide. Also known as the "silent killer", CO can kill before you are aware you are being poisoned. According to the Partnership for Clean Indoor Air, approximately 2 billion people worldwide are at risk and an estimated 1.6 million premature deaths occur worldwide each year due to CO poisoning.
Dr. Leo Latasch, Medical Director for the Frankfurt am Main EMS, commented: "With the Rad-57, our paramedics just slip the noninvasive finger sensor on the patient and press a button. Within seconds they can see the carbon monoxide and oxygen saturation levels in the blood, as well as pulse rate, right there on-the-spot—without having to draw a single drop of blood."
Masimo Founder and CEO, Joe Kiani, stated, "The decision to equip every fire vehicle in the city with Masimo Rad-57s shows that Frankfurt has an unwavering commitment to protect both their citizens and their own EMS professionals from the devastating short and long-term effects of CO poisoning. We commend their leadership in establishing a city-wide policy for CO screening by the Masimo rainbow Rad-57."
About Frankfurt am Main Emergency Medical Services
Frankfurt am Main is a very busy and densely populated city that is home to a number of international companies and 229 bank institutions, including the Central Bank of Germany and the European Central Bank, and the largest European airport. Frankfurt am Main EMS is facilitated by the Frankfurt fire department in partnership with several health organizations, like the Red Cross, to provide ambulance services. The dispatch center coordinates 30-35 ambulances and one helicopter within a 249 square kilometer area—processing approximately 85,000 emergency calls per year.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Rad-57 Pulse CO-Oximeters will help paramedics to noninvasively measure carbon monoxide levels within seconds for all patients, risks related to our assumptions that using the Rad-57 will help to protect the health and safety of Frankfurt citizens and paramedics from the dangers of undetected CO poisoning, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Prof. Dr. Leo Latasch
Frankfurt am Main Emergency Medical Services
+49 69 212 33813
leo.latasch@stadt-frankfurt.de
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Premier Members to Benefit from Full Line of Market-Leading Masimo rainbow Pulse CO-Oximetry Solutions with New Agreement
Irvine, California – August 19, 2010 –Masimo (NASDAQ: MASI), the inventor of Masimo rainbow® Pulse CO-Oximetry™, Masimo rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, announces an expanded supplier agreement that adds Masimo rainbow Pulse CO-Oximetry and patient monitoring solutions to the purchasing contract with Premier Purchasing Partners, LP, the group purchasing unit of Premier, Inc. Now, in addition to Masimo SET pulse oximetry solutions, members of Premier, a leading national healthcare group purchasing organization, will be able to directly access and purchase the complete market-leading line of Masimo rainbow Pulse CO-Oximeters and sensors at significant contracted savings.
Effective August 1, 2010, through May 31, 2012, Premier members can purchase Masimo standalone (Radical-7®, Rad-87®) and portable (Rad-57®, Pronto-7™) rainbow Pulse CO-Oximeters on contract, along with ReSposable, adhesive/disposable, and reusable sensors for all categories of patients (from neonatal and infant/pediatric to adult).
With Masimo rainbow Pulse CO-Oximeters, clinicians can noninvasively and continuously measure many blood constituents that previously required invasive procedures, including: hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the gold-standard performance of Measure-Through Motion and Low Perfusion Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate. Featuring revolutionary rainbow technology, these advanced patient monitors provide continuous measurements of critical physiological parameters with real-time tracking and trending capabilities that help clinicians to identify abnormalities and patient deterioration sooner—facilitating earlier detection and treatment of potentially life-threatening conditions.
And, as the only upgradable oximetry technology platform enabling new device capabilities to be added through simple field-installed software upgrades, Masimo rainbow Pulse CO-Oximeters allow healthcare facilities to fight the rising costs of equipment obsolescence. Instead of costly device and hardware replacements, new noninvasive measurements and patient monitoring capabilities can be cost-effectively added to existing "rainbow-ready" Masimo oximeters via a quick and easy field-installed software upgrader tool.
Masimo Founder & CEO, Joe Kiani, stated, "Masimo rainbow Pulse CO-Oximeters provide clinicians with breakthrough noninvasive measurements and physiological information they've never before had continuous, real-time access to. Armed with this level of clinical detail allows clinicians to improve and reduce the cost of care."
About the Premier Healthcare Alliance
Premier is a performance improvement alliance of more than 2,400 U.S. hospitals and nearly 70,000 other healthcare sites working together to achieve high quality, cost-effective care. Owned by not-for-profit hospitals, Premier maintains the nation's most comprehensive repository of clinical, financial and outcomes information and operates a leading healthcare purchasing network. A world leader in helping deliver measurable improvements in care, Premier works with the Centers for Medicare & Medicaid Services and the United Kingdom's National Health Service North West to improve hospital performance. Headquartered in Charlotte, N.C., Premier also has offices in San Diego, Philadelphia and Washington. http://www.premierinc.com.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the specificity and sensitivity of Masimo rainbow Pulse CO-Oximetry technology will provide continuous measurements of critical physiological parameters with real-time tracking and trending capabilities in all patients; risks related to the assumption that continuous Pulse CO-Oximetry measurements will enable clinicians to identify patient deterioration sooner and facilite earlier detection and treatment of potentially life-threatening conditions, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Arrowhead Regional Medical Center Converts to Masimo rainbow Technology for Advanced Noninvasive Patient Monitoring Capabilities
Southern California Acute Care Facility Standardizes System-wide to Improve Patient Care
Irvine, California – August 12, 2010 – Arrowhead Regional Medical Center (ARMC) in Colton, California, and Masimo (NASDAQ: MASI), the inventor of Masimo rainbow Pulse CO-Oximetry™, Masimo rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, today jointly announced the completion of ARMC's system-wide conversion to Masimo rainbow technology. The conversion ensures that all ARMC patients can be monitored using the most technologically and clinically-advanced noninvasive patient monitoring technologies available—providing real-time measurement results for critical physiological parameters that help clinicians to more rapidly assess, diagnose, and treat patients.
"The decision to convert our network to the Masimo rainbow technology platform came down to wanting to equip our facility with the best and most advanced noninvasive patient monitoring capabilities available," stated Patrick Petre, Director at Arrowhead Regional Medical Center. "Our mission is to provide quality care to the community using the most advanced patient care technology."
The system-wide conversion standardizes all of ARMC's sites of care to Masimo rainbow technology—the only upgradable pulse oximetry platform that allows hospitals to add breakthrough noninvasive blood constituent, hemodynamic, and respiration measurement capabilities that previously required invasive procedures. The ability to continuously and noninvasively measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to Masimo SET 'gold standard' Measure-Through Motion and Low Perfusion oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR) measurements enables monitoring of multiple physiological parameters simultaneously—facilitating earlier detection and treatment of life-threatening conditions. The conversion involved upgrading virtually all of the hospital's multiparameter patient monitors, oximeters, and sensors to enable the new patient monitoring capabilities.
"This upgrade is important because it enables us to now provide noninvasive, technologically-advanced monitoring technologies to our patients," said Dr. David T. Wong, a surgeon and chief of Trauma and Critical Care Services at ARMC. "It also provides flexibility in that we can now continuously monitor blood constituents, fluid responsiveness, and advanced oxygen delivery parameters, in addition to the more common measurements of arterial saturation and pulse rate."
Masimo Founder and CEO, Joe Kiani, stated, "Arrowhead Regional Medical Center joins a growing list of the world's most demanding hospitals standardizing to Masimo rainbow as their universal oximetry solution. Offering the most advanced noninvasive measurement capabilities, our rainbow oximetry platform helps state-of-the-art hospitals leverage cutting-edge medical technologies that improve patient outcomes and reduce the cost of care."
About Arrowhead Regional Medical Center
Arrowhead Regional Medical Center is a state-of-the-art 456-bed acute care facility featuring the newest technology in the field of patient care. The Medical Center is the host to a 24-hour Emergency Department, Level II Trauma Center, three Family Health Centers and the only Burn Center serving San Bernardino, Riverside, Inyo and Mono counties. For more information, visit: www.arrowheadmedcenter.org
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET and Masimo rainbow oximetry technologies will provide sufficient sensitivity and specificity to accurately detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, risks related to our belief that Masimo SET and rainbow measurements will help clinicians guide treatment options, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Jorge Valencia
Arrowhead Regional Medical Center
(909) 580-6180
valenciaj@armc.sbcounty.gov
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


San Antonio Community Hospital Converts to Masimo rainbow Technology for Advanced Noninvasive Patient Monitoring Capabilities
Southern California Acute Care Facility Standardizes System-wide to Improve Patient Care
Irvine, California – August 10, 2010 – San Antonio Community Hospital (SACH) in Upland, California, and Masimo (NASDAQ: MASI), the inventor of Masimo rainbow Pulse CO-Oximetry™, Masimo rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, today jointly announced the completion of San Antonio Community Hospital's system-wide conversion to Masimo rainbow technology. The conversion ensures that all SACH patients will be monitored using the most technologically and clinically-advanced noninvasive patient monitoring technologies available—providing real-time measurement results for critical physiological parameters that help clinicians to more rapidly assess, diagnose, and treat patients.
"We converted our entire network to Masimo's advanced oximetry technology platform to give our clinical team the superior tools they need to provide the best clinical care possible and, after careful evaluation, it became clear that we would also be lowering our overall costs by implementing Masimo rainbow SET," stated Steven Moreau, President and CEO of San Antonio Community Hospital. "Staying on the forefront of medical technology advancements is just one of the ways SACH is staying true to our mission of providing outstanding healthcare in the community."
The system-wide conversion standardizes all of SACH's sites of care, including 279 hospital beds, to Masimo rainbow technology—the only upgradable pulse oximetry platform that allows hospitals to add breakthrough noninvasive blood constituent, hemodynamic, and respiration measurement capabilities that previously required invasive procedures. The ability to continuously and noninvasively measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to Masimo SET Measure-Through Motion and Low Perfusion oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR) measurements enables monitoring of multiple physiological parameters simultaneously—facilitating earlier detection and treatment of life-threatening conditions. The conversion involved upgrading virtually all of the hospital's multiparameter patient monitors, oximeters, and sensors to enable the new patient monitoring capabilities.
Perry Chu, MD, Anesthesiologist at San Antonio Community Hospital, stated, "Masimo's oximeters have proven to be the most sensitive and reliable, picking up saturation levels even in low perfusion states and when the patient is cold. I am particularly impressed with the Radical-7's ease-of-use, portability, and range of clinical utility as it affords us the unique ability to noninvasively monitor hemoglobin levels more closely and tightly."
Masimo Founder and CEO, Joe Kiani, stated, "San Antonio Community Hospital's conversion to the Masimo rainbow technology platform is proof positive of their commitment to offering the most advanced patient care throughout the Inland Empire."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET and Masimo rainbow SET oximetry technologies will provide sufficient sensitivity and specificity to accurately detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, risks related to our belief that Masimo technologies and measurements will help clinicians to more rapidly assess, diagnose, and treat patients, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Lisa Ciccanti
San Antonio Community Hospital
(909) 920-4740
lciccanti@sach.org
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Delaware Becomes the First to Equip Every Fire District in the State with Most Advanced Noninvasive Carbon Monoxide Screening Technology
150 Handheld Masimo Rad-57 Pulse CO-Oximeters Will Be Distributed to Fire Districts at August 6-7 Event
Irvine, California – August 5, 2010 – Odessa Fire Company in the State of Delaware and Masimo (NASDAQ: MASI), the inventor of Masimo rainbow Pulse CO-Oximetry™, Masimo rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, today jointly announced that 150 Masimo Rad-57® Pulse CO-Oximeters will be distributed to every fire district in the state of Delaware during a statewide training event at the main fire station (304 Main Street in Odessa) on August 6-7, 2010.
Delaware is the first state in the country to place this lifesaving technology in every fire response district. The Rad-57 Pulse CO-Oximeters, which noninvasively measure the amount of carbon monoxide (CO) in the bloodstream, were purchased to enhance the response capabilities of Delaware's first responders and protect the health and safety of the public and emergency response personnel from the dangers of undetected CO poisoning. Dave Aber, EMS Supervisor at the Odessa Fire Company, stated, "With 150 Rad-57s hitting the streets with our first responders, we're potentially the first state in the nation to become fully compliant with new NFPA 1584 national standards for Firefighter Rehabilitation and Medical Monitoring."
Carbon monoxide is an odorless, colorless, toxic gas that is impossible to see, taste or smell. Also known as the "silent killer", CO can kill before you are aware you are being poisoned. The leading cause of accidental poisoning deaths in the U.S., CO kills nearly 500 each year and sends another 20,000 people to hospital emergency rooms for treatment each year and, according to the Centers for Disease Control (CDC), cases of CO poisoning are on the rise—climbing 36% between 2001 and 2006.1
An 18-year EMS veteran, Aber says that the Rad-57 is the best tool they have in the fight against this rise in CO poisoning, "Rad-57 is the fastest, easiest, most accurate way for us to determine at the scene of an emergency call if a person is CO poisoned. By simply placing a noninvasive finger sensor on the patient and pressing a button, we can measure CO and oxygen saturation levels in the blood within seconds. In emergencies, when seconds can mean a lifesaving difference, Rad-57 takes the guesswork out and provides us with the certainty we need to immediately identify who is CO poisoned and how severely, so we can initiate the right treatment at the right time."
Funding for the Rad-57s was made possible through a $420,000 federal grant awarded to the Odessa Fire Company by the Assistance to Firefighter Grant program through the Department of Homeland Security. "It's great news," stated Delaware Congressman Mike Castle. "The sacrifices made by the volunteer firefighters across the state can never be repaid, but we should always work together to support first responders."
Masimo Founder and CEO, Joe Kiani, stated, "This statewide initiative to equip every fire district with our Rad-57s arms Delaware first responders with the technology they need to protect themselves and the public from the devastating short- and long-term effects of CO poisoning. Together, the Odessa Fire Company and the state of Delaware have shown outstanding public safety leadership at a time when it's needed most—as CO poisoning statistics continue to mount."
1 Centers for Disease Control and Prevention (CDC) Morbidity and Mortality Weekly Report (MMWR), August 22, 2008: Nonfatal, Unintentional, Non--Fire-Related Carbon Monoxide Exposures--United States, 2004—2006 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5733a2.htm?s_cid=mm5732a2_e
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Rad-57 Pulse CO-Oximeters will help first responders to noninvasively measure carbon monoxide levels within seconds for all patients, risks related to our assumptions that using the Rad-57 will help to protect the health and safety of Delaware citizens and first responders from the dangers of undetected CO poisoning, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
David Aber, NREMT-P
Odessa Fire Company
(302) 740-7661
medic613@comcast.net
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports Second Quarter 2010 Financial Results
Total quarterly revenue exceeds $100 million for first time in company's history
Q2 2010 Highlights (compared to Q2 2009):
- Total revenue rose 20% to $100.1 million
- Product revenue rose 26% to $88.0 million
- Masimo SET and Masimo Rainbow SET unit shipments rose 35% to 36,700
- Rainbow revenue rose 60% to $7.2 million
- GAAP EPS of $0.24 included $0.01 in one-time expenses. Adjusted EPS of $0.25, compared to $0.22 in year-ago period
Irvine, California, August3, 2010 – Masimo Corporation (NASDAQ: MASI), today announced its financial results for the second quarter of 2010.
Masimo's total revenue for the second quarter rose 19.8% to $the second quarter, comp100.1 million, compared to $83.6 million for the second quarter of 2009. Masimo's second quarter product revenue rose 25.6% to $88.0 million, compared to $70.0 million for the second quarter of 2009. Revenue from Masimo Rainbow SET products rose 59.5% to $7.2 million in ared to $4.5 million for the second quarter of 2009.
Net income for the second quarter was $14.3 million, or $0.24 per diluted share, including $0.01 per diluted share in one-time marketing-related expenses that Masimo had previously planned and announced after receiving $30.1 million in proceeds from an antitrust lawsuit against Covidien in the first quarter of 2010. Excluding these one-time expenses, adjusted net income for the second quarter was $15.2 million, or $0.25 per diluted share, compared to net income of $13.1 million, or $0.22 per diluted share, in the second quarter of 2009.
During the second quarter, the company shipped 36,700 Masimo SET pulse oximetry and Masimo Rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 35.4% compared to 27,100 in the same period last year. Masimo estimates its worldwide installed base as of July 3, 2010 to be 789,000 units, up 16.9% from 675,000 as of July 4, 2009.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Masimo's second quarter results reveal momentum across virtually all parts of our business, demonstrating the underlying strength and value of our technology to clinicians and patients. Our core SET franchise grew by double digits again this quarter and revenue from Rainbow SET products achieved a new high, allowing Masimo to deliver our first-ever $100 million revenue quarter. Our second quarter performance reflects strong domestic and international customer demand from both our direct and OEM sales channels. The quarter was also highlighted by the launch of two new Masimo innovations: Rainbow Acoustic Monitoring for continuous and noninvasive respiration rate monitoring, and Pronto-7, a palm-sized device for noninvasive spot-check hemoglobin testing."
As of July 3, 2010, cash, cash equivalents and short-term investments totaled $111.0 million, compared to $189.0 million as of January 2, 2010. The decline was due primarily to the March 31, 2010 dividend payment of $117.5 million, partially offset by the net proceeds from the antitrust lawsuit and operating cash flow in the first six months of 2010.
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation code for both dial-in numbers is 88305128. A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. After the live webcast, the call will be available on Masimo's website through September 3, 2010. In addition, a telephonic replay of the call will be available through August 17, 2010. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 88305128.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Rainbow Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com. Any information contained in, or that can be accessed through, our website is not incorporated by reference into, nor is it in any way a part of, this release.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo Rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any new or existing products and technologies of ours; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.
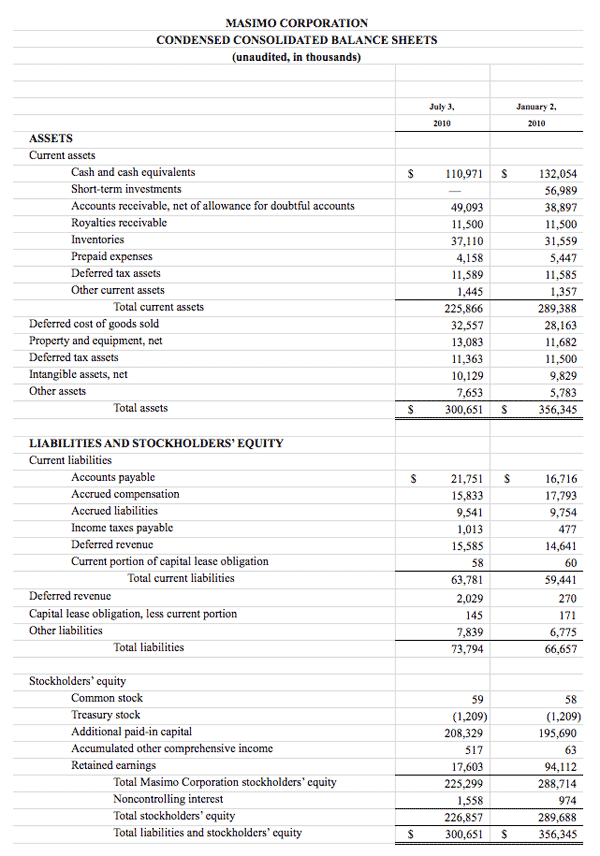
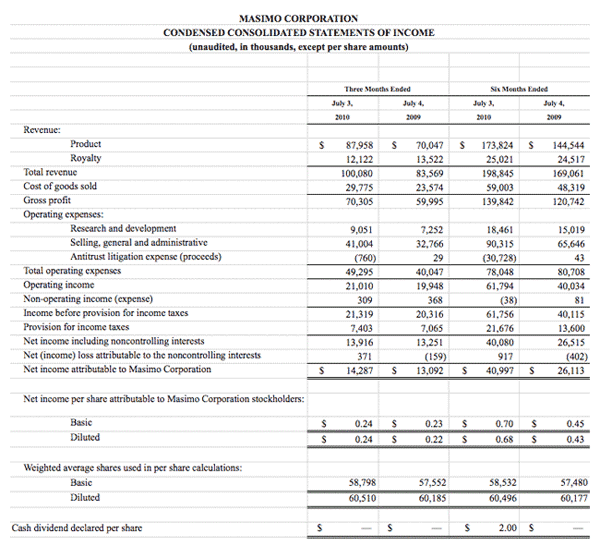


Amerinet Members to Benefit from Masimo's Full Line of Market-Leading Pulse CO-Oximetry Solutions with New Agreement
Irvine, California – July 26, 2010 – Amerinet Inc., a leading national healthcare group purchasing organization, and Masimo (NASDAQ: MASI), the inventor of Masimo Rainbow Pulse CO-Oximetry™, Masimo Rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, jointly announce a new supplier agreement—offering Amerinet members significant contracted savings on Masimo's complete market-leading line of Pulse CO-Oximeters and sensors.
Effective July 1, 2010, through June 30, 2013, Amerinet members can purchase Masimo standalone (Radical-7®, Rad-87®) and portable (Rad-57®, Pronto-7™) Pulse CO-Oximeters on contract, along with reusable and disposable sensors for all categories of patients (from neonatal, infant/pediatric to adult).
With Masimo Pulse CO-Oximeters, clinicians can noninvasively and continuously measure many blood constituents that previously required invasive procedures, including: hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the gold-standard performance of Measure-Through Motion and Low Perfusion Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate. Featuring revolutionary Masimo Rainbow SET® Pulse CO-Oximetry™ technology, these advanced patient monitors provide continuous measurements of critical physiological parameters with real-time tracking and trending capabilities that help clinicians to identify patient deterioration sooner—facilitating earlier detection and treatment of potentially life-threatening conditions.
About Amerinet
As a leading national healthcare group purchasing organization, Amerinet strategically partners with acute and alternate care providers to reduce costs and improve quality through its performance solutions. Built on a foundation of data, savings and trust, and supported by a team of clinical and supply chain experts, Amerinet enriches healthcare delivery for its members and the communities they serve. To learn more about the Amerinet difference, visit www.amerinet-gpo.com.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Rainbow Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the specificity and sensitivity of Masimo Rainbow Pulse CO-Oximetry technology will provide continuous measurements of critical physiological parameters with real-time tracking and trending capabilities in all patients; risks related to the assumption that continuous Pulse CO-Oximetry measurements will enable clinicians to identify patient deterioration sooner and facilite earlier detection and treatment of potentially life-threatening conditions, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Evan Danis
Amerinet
(724) 778-3423
evan.danis@amerinet-gpo.com
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo and Hygia Settle Lawsuit
Irvine, California – July 22, 2010 – Masimo (NASDAQ: MASI), the inventor of Masimo Rainbow Pulse CO-Oximetry™, Masimo Rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, announced today it has settled a lawsuit with Hygia Health Services, a reprocessor of medical devices, on mutually agreeable terms. Terms of the settlement agreement remain confidential.
The lawsuit had been pending in the United States District Court for the Northern District of Alabama since May 2009.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Rainbow Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report Second Quarter 2010 Financial Results after Market Close on August 3, 2010
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., July 20, 2010 -- Masimo (NASDAQ: MASI), the inventor of Masimo Rainbow Pulse CO-Oximetry™, Masimo Rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, today announced it will release second quarter 2010 financial results for the period ended July 3, 2010, after the market closes on August 3, 2010. A conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer. A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation code for both dial-in numbers is 88305128. After the live webcast, the call will be available on Masimo's website through September 3, 2010. In addition, a telephonic replay of the call will be available through August 17, 2010. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 88305128.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Rainbow Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Acquires SedLine and Enters the Brain Function Monitoring Market
Acquisition Allows Masimo to Expand Clinician Access to Innovative SedLine® Technology
Irvine, California – July 13, 2010 – Masimo (NASDAQ: MASI), the inventor of Masimo Rainbow Pulse CO-Oximetry™, Masimo Rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, today announced that it has acquired SedLine, Inc., a pioneering manufacturer of brain function monitoring. The acquisition of SedLine formalizes Masimo's commercial entry into the brain function monitoring market—enabling the innovative SedLine technology to become more widely available to clinicians in both U.S. and international healthcare markets. Terms of the acquisition were not disclosed.
Masimo Founder and CEO, Joe Kiani, stated, "Multiple leading hospitals in the U.S. have already chosen SedLine because they believe, as Masimo does, that SedLine's technology and performance offers an advancement over other brain function monitoring technologies. Masimo has always been a strong advocate for innovation, patient care, and choice in the healthcare marketplace. We are happy to integrate SedLine into our product pipeline to continue the Masimo tradition of offering groundbreaking noninvasive medical technologies that significantly improve patient outcomes and reduce the cost of care."
Brain function monitoring has become a widely utilized modality for assessing depth of anesthesia and sedation. Based primarily on analysis of the electroencephalogram (EEG), brain function monitors continuously measure the electrical activity of the brain and translate those changes to the level of consciousness. Brain function monitoring has been deployed in a variety of clinical environments including the operating room to manage depth of sedation, the intensive care units where patients can be sedated for prolonged periods, and for ambulatory units where large numbers of patients undergo short duration procedures.
Privately-held and backed with a financial investment from Masimo last year, SedLine was formed with the mission to advance brain function monitoring to improve the care of patients under anesthesia or sedation. SedLine's technology is based on 10 years of innovation that delivers proven 4-channel EEG integrated algorithm performance, demonstrated reliability under challenging clinical conditions, and superior resistance to electrocautery1— offering a cost-effective alternative to other brain function monitoring technologies.
Terri Monk, MD, Professor of Anesthesiology at Duke University Hospital, commented, "In my experience, SedLine monitors provide valuable and reliable clinical information that helps me to deliver optimal anesthesia care. I am pleased but not surprised that SedLine would become part of Masimo, as they both are technology-driven organizations committed to improving patient care."
Leslie Jameson, MD, Associate Professor and Vice Chair, University of Colorado, Department of Anesthesiology added, "When I need processed EEG monitoring to optimize anesthesia care in our operating rooms, I opt for SedLine. The 4-channel EEG screen provides a diagnostic quality display that can be optimized for each patient. When combined with the DSA screen, the SedLine is a very valuable tool to optimize anesthetic management, particularly in certain patient groups. I look forward to seeing how Masimo will expand SedLine's technology platform to further enhance its already considerable clinical value."
Masimo and SedLine are in the process of developing a full integration plan and will provide details when they are finalized. In the meantime, current or new SedLine customers should continue to contact SedLine personnel for any ordering or other customer service inquiries at (888) 733-5467.
1White PF, Tang J, Ma H, Wender RH, Sloninsky A, Kariger R. "Is the Patient State Analyzer with the PSArray2 a Cost-effective Alternative to the Bispectral Index Monitor During the Perioperative Period?" Anesth Analg. 2004;99:1429-1435.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Rainbow Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the acquisition of SedLine will enable Masimo to commercially enter the brain function monitoring market and make SedLine technology more widely available to clinicians in both U.S. and international healthcare markets; risks related to our assumptions regarding product integration, acquisition benefits, and the market timing of commercial products featuring SedLine technology, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SedLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Receives FDA Clearance for Pronto-7 Making the Revolutionary Palm-sized Device for Noninvasive Hemoglobin Spot-Check Testing Available to U.S. Healthcare Providers
Irvine, California – June 28, 2010 – Masimo (NASDAQ: MASI), the inventor of Masimo Rainbow Pulse CO-Oximetry™, Masimo Rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, today announced FDA 510(k) clearance for the Pronto-7™—a new handheld device designed for quick and easy noninvasive hemoglobin (SpHb®)
 |
| The palm-sized Masimo Pronto-7™ offers noninvasive hemoglobin results in under one minute. |
spot-check testing, along with SpO2, pulse rate, and perfusion index, in virtually any environment.
Masimo Founder and CEO, Joe Kiani, stated: "We are happy that in addition to the CE Marking we now have FDA clearance for Pronto-7, allowing U.S. clinicians and patients to also benefit from the latest technology for noninvasive hemoglobin spot-check testing."
Dr. Anunciacion Andro, a busy primary care physician in Hesperia, California, says needle-less, pain-free hemoglobin testing has big patient care benefits: "The ability to get noninvasive hemoglobin spot-check results has been a great benefit to my patients—who are happy to not be stuck with a needle."
Dr. Aditya Chopra, a private practice internist in Annapolis, MD, confirmed the value of fast, point-of-care spot-check testing in his practice, saying: "Noninvasive hemoglobin spot-check testing has increased the efficiency of my practice by reducing the time to take blood, send it to the lab, and call patients back with their results."
A low hemoglobin level is called anemia, a pervasive blood disorder that, according to the World Health Organization (WHO), affects 1.6 billion people worldwide and causes one million deaths a year. A top-ten risk factor contributing to the global burden of disease, anemia results in lost cognitive function and productivity that is estimated to cost $50 billion annually in gross domestic product (GDP) losses worldwide. Chronic anemia is characterized by consistently low hemoglobin levels that can be the result of a diet deficiency or illness such as cancer. Acute anemia is a sudden drop in hemoglobin levels that can result from internal or external bleeding due to surgery or trauma.
According to Aryeh Shander, M.D., President-Elect of the Society for the Advancement of Blood Management (SABM) and the Executive Medical Director for The Institute for Patient Blood Management & Bloodless Medicine and Surgery at Englewood Hospital and Medical Center in Englewood, New Jersey, "Noninvasive hemoglobin testing at the point-of-care offers a giant leap forward in our ability to tackle the global burden of anemia. Although it is a common blood disorder, it is grossly under-tested and under-diagnosed. And, if left untreated, anemia has a variety of serious health consequences and complications. The beauty of immediate, noninvasive hemoglobin testing is that it will allow more patients to be assessed, so their physician can determine additional test options and initiate potentially lifesaving treatment."
Hemoglobin is one of the most commonly ordered tests in both hospital and non-hospital settings because it is critical to assessing anemia and blood loss. However, traditional lab testing requires a painful needle stick for the patient, time-consuming blood draws for the clinician, and typically provides delayed results. The palm-sized Pronto-7—with dimensions of just 5.1" x 2.8" x 1" and weight of 10.5 ounces— represents a breakthrough solution for measuring hemoglobin in less than one minute. It puts the power of noninvasive hemoglobin spot-check testing into any clinician's hands in almost any environment—without the needles, time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests.
Pronto-7 features embedded 802.11 b/g and Bluetooth communication capabilities that make wireless printing and emailing of test results quick and easy and future capabilities enabling wireless transmission to electronic health record (EHR) systems are planned. It is also the first Masimo device to feature Rainbow 4D™—designed for fast and accurate spot-check SpHb measurements, along with SpO2 and pulse rate, even in low perfusion conditions.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumption that Masimo noninvasive and continuous total hemoglobin (SpHb) will provide faster, easier and safer means for measuring total hemoglobin over alternative hemoglobin measurement methods, risks related to our belief that Masimo Pronto-7 enables clinicians to simply, quickly, and painlessly assess their patients' hemoglobin status to uncover hidden health dangers and potentially life-threatening conditions, risks related to our assumptions that the availability of Pronto-7 will lead to additional device sales, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57, Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Initiates Limited Market Release of Pronto-7™—New Revolutionary Palm-sized Device for Noninvasive Hemoglobin Spot-Check Testing at the Point-of-Care
Allows Physician Offices, Emergency Medical Services, Blood Donation Centers, Clinics, and Healthcare Facilities to Measure Hemoglobin in Under a Minute—Without Removing a Drop of Blood
Irvine, California – June 23, 2010 – Masimo (NASDAQ: MASI), the inventor of Rainbow Pulse CO-Oximetry™, Rainbow Acoustic Monitoring™, and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, today announced the international limited market release of Masimo Pronto-7—a new handheld device designed for quick and easy noninvasive hemoglobin (SpHb®) spot-check testing, along with SpO2, pulse rate and perfusion index, in virtually any environment. As part of the limited market release, select physician offices, emergency medical services, blood donation centers, and hospitals in eligible countries outside the United States will be the first to use and benefit from this new point-of-care blood testing capability.
 |
| The palm-sized Masimo Pronto-7™ offers noninvasive hemoglobin results in under one minute. |
Hemoglobin is one of the most commonly ordered tests in both hospital and non-hospital settings because it is critical to assessing anemia and blood loss. However, traditional lab testing requires a painful needle stick for the patient, time-consuming blood draws for the clinician, and typically provides delayed results. The Pronto-7 represents a breakthrough solution for measuring hemoglobin in less than one minute—without the needles, time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests. The Pronto-7 is also the first Masimo device to feature Rainbow 4D™—designed for fast and accurate spot-check SpHb measurements – along with SpO2 and pulse rate - even in low perfusion conditions.
The palm-sized Masimo Pronto-7—with dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces)—puts the power of noninvasive hemoglobin spot-check testing into any clinician's hands in almost any environment. Because of the device's embedded 802.11 b/g and Bluetooth communication capability, wireless printing or emailing of test results is enabled and future upgrades will allow for wireless transmission to electronic health record (EHR) systems.
A low hemoglobin level is called anemia, a pervasive blood disorder that, according to the World Health Organization (WHO), affects two billion people worldwide and causes one million deaths a year. A top ten risk factor contributing to the global burden of disease, anemia results in lost cognitive function and productivity that costs $50 billion annually in gross domestic product (GDP) losses worldwide. Anemia can be chronic or acute. Chronic anemia is characterized by consistently low hemogl obin levels that can be the result of a diet deficiency or illness such as cancer. Acute anemia is characterized by a sudden drop in hemoglobin levels that can result from internal or external bleeding due to surgery or trauma.
Masimo Founder and CEO, Joe Kiani, stated: "The portable Pronto-7 device is a revolutionary new product that offers speed, functionality, simplicity, and accuracy in a completely noninvasive manner. It's the perfect complement to existing Masimo Rainbow devices currently used in hospitals throughout the world to help clinicians continuously assess hemoglobin, detect internal bleeding, and aid in blood transfusion management. We believe that the Pronto-7 will have significant appeal to clinicians whose patients don't require continuous hemoglobin monitoring, but do appreciate the immediate and noninvasive anytime/anywhere testing capability it offers."
With CE Marking and the international limited market release underway, the Pronto-7 can now be demonstrated and quoted by Masimo direct and distributor representatives in Europe, Asia, Canada, Australia, and South America.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumption that Masimo noninvasive and continuous total hemoglobin (SpHb) will provide faster, easier and safer means for measuring total hemoglobin over alternative hemoglobin measurement methods, our belief that Masimo Pronto-7 enables clinicians to simply, quickly, and painlessly assess their patients' hemoglobin status on-the-spot to uncover hidden health dangers and potentially life-threatening conditions, and our assumptions that the availability of Pronto-7 will lead to additional device sales, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57, Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Study Presented at European Society of Anaesthesia Validates Masimo Noninvasive and Continuous Hemoglobin Monitoring
Irvine, California – June 16, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that a new independent study demonstrating the clinical accuracy of Masimo noninvasive and continuous hemoglobin (SpHb) monitoring was presented this week at the European Society of Anaesthesiology (ESA) Annual Congress in Helsinki, Finland.
In the study, titled "Comparison Between a New Noninvasive Continuous Technology of Spectrophotometry-based and RBC Count for Haemoglobin Monitoring During Surgery with Hemorrhagic Risk," Dr. Lionel Lamhaut and researchers from the Department of Anesthesiology and Intensive Care at Necker University Hospital in Paris, France, compared the accuracy of Masimo SpHb with hemoglobin measurements obtained invasively via laboratory blood analysis in 20 patients undergoing high blood-loss surgery. SpHb and invasive hemoglobin measurements were simultaneously recorded at the beginning and end of any clinical intervention and before and after blood transfusion. Results showed a strong agreement between the two (0.88), with a bias of 0.26 g/dL and standard deviation of 1.1 g/dL—leading researchers to conclude that this study, conducted under real-world clinical conditions, "confirms the first tests realized by the manufacturer." Affirming the clinical accuracy and utility of Masimo SpHb, researchers further noted that "the correlation is good, suggesting the possibility of a daily use of this technology."1
Masimo SpHb is available as part of Masimo Rainbow platform—the first-and-only platform to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI).
1 L. Lamhaut, R. Apriotesei, M. Lejay, B. Vivien, P. Carli. "Comparison Between a New Noninvasive Continuous Technology of Spectrophotometry-based and RBC Count for Haemoglobin Monitoring During Surgery with Hemorrhagic Risk" Eur J Anaesthesiol 2010; 27 (Suppl 47): 3AP7-1.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of respiration rate with an easy-to-use acoustic adhesive sensor (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpHb and PI will provide sufficient sensitivity and specificity to facilitate continuous measurement capabilities in all patients and deliver clinical improvement over alternative hemoglobin and perfusion assessment methods to allow for further adoption of the technology; and other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Initiates Full Market Release of Rainbow Acoustic Monitoring
First Respiration Rate Monitor Offering Accuracy, Ease of Use, and High Patient Tolerance
Irvine, California – June 7, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today the full market release of its latest medical technology breakthrough—Masimo Rainbow Acoustic Monitoring™— providing continuous and noninvasive respiration rate (RRa™) that is accurate, easy-to-use, and enhances patient tolerance. With full commercial availability to hospitals worldwide, Masimo Rainbow Acoustic Monitoring is expected to have a significant impact on the general care floor and during procedural sedation by helping clinicians detect respiratory compromise and patient distress earlier.
Respiration rate is an important vital sign and is especially important for post-surgical patients receiving patient-controlled analgesia (PCA) for pain management as sedation can induce respiratory depression and place patients at considerable risk of serious injury or death.1-4 Although the Anesthesia Patient Safety Foundation (APSF) recommends continuous oxygenation and ventilation monitoring in all patients receiving opioids,5,6 current methods for respiration rate monitoring are limited by reliability or patient tolerance.
"We now have an accurate monitor that continuously displays respiratory rate—the neglected vital sign—from a sensor that is unnoticeable to the patient," stated Michael Ramsay, M.D., Chief of the Department of Anesthesiology and Pain Management at Baylor University Medical Center in Dallas, Texas. "The digital signal is transmitted to the care giver and serves as an early warning signal of respiratory compromise."
Marilyn Nemerever, RN, Director of Acute Care at Swedish Medical Center in Seattle, Washington, stated, "Our success with Rainbow Acoustic Monitoring and the way it allows our clinicians to identify patients in distress earlier and then make the necessary clinical interventions has led us to expand its use, along with Patient Safety Net, to other care areas within the hospital system. Implementation of these key technologies have greatly contributed to the culture of safety at Swedish Medical Center."
 |
|
| (Photo, Left) The Rainbow Acoustic Sensor is a low-profile adhesive sensor that is easy to apply and comfortable to wear. (Photo, Above) The acoustic waveform allows clinicians to view the signal quality and rate. |
Masimo Founder and CEO, Joe Kiani, stated, "The excellent feedback we received from hospital clinicians during the limited market release demonstrates that Masimo Rainbow Acoustic Monitoring addresses a longstanding and growing need to more accurately and easily monitor patient breathing. The full market release of Rainbow Acoustic Monitoring means that hospitals around the world now have a new tool to significantly improve patient safety and decrease the cost of care."
Masimo Rainbow Acoustic Monitoring is currently available through Masimo direct sales representatives and authorized distributors worldwide. For sales inquiries, please email sales@masimo.com or visit: https://professional.masimo.com/technology/ventilation-and-respiration/rra/.
1 Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Alert: Patient controlled analgesia by proxy. Chicago: JCAHO, 2004
2 Institute for Safe Medication Practice. Safety issues with patient-controlled analgesia: Part 1-How errors occur. Huntingdon Valley: ISMP, 2003
3 Institute for Safe Medication Practices. Safety issues with patient-controlled analgesia: Part II – How to prevent errors Huntingdon: ISMP, 2003
4 Bird M. Acute pain management: a new area of liability for anesthesiologistsinger MB. Dangers of Postoperative Opioids: APSF Woop and White paper address prevention of postoperative respiratory complications. APSF Newsletter, 2006;21:61–7. http://www.apsf.org/assets/Documents/winter2007.pdf
6 Stoelting RK, Weinger MB. Dangers of Postoperative Opioids—Is There a Cure? APSF Newsletter, 2009;24:25-26.
http://www.apsf.org/assets/Documents/summer2009.pdf
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results and performance of Masimo Rainbow Acoustic Monitoring; our belief that the breakthrough respiration rate monitoring capabilities of Masimo's proprietary technology will provide sufficient sensitivity and specificity to measure respiratory rate in a variety of patients and monitoring conditions, enabling clinicians to detect and treat respiratory compromise and patient distress earlier; as well as assumptions regarding full commercialization timing and worldwide market availability of the technology; and other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at William Blair & Company 30th Annual Growth Stock Conference
IRVINE, Calif. – June 4, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that its management is scheduled to present at the William Blair & Company 30th Annual Growth Stock Conference at The Four Seasons Hotel in Chicago on Wednesday, June 16, at 10:00 a.m. Central Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Study Finds Newborn Screening with Masimo SET Pulse Oximetry Increases Detection of Congenital Heart Disease
Researchers Show Multiple Defects Would Have Been Missed without Masimo SET
Irvine, California – June 3, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that a new clinical study published online in the German pediatric journal, Klinische Pädiatrie, showed that adding Masimo SET pulse oximetry screening to the physical examination of newborns was an effective method to achieve early diagnosis of critical congenital heart disease (CHD).1
CHD affects five to ten of every 1,000 newborns, resulting in 3% of all infant mortalities. Improving early detection and treatment is critical because up to 30% of all CHD-related deaths in the first year of life are due to failure to detect the condition. However, critical duct-dependent CHD is often present without abnormal auscultation (listening to sounds within the body). In total, fewer than 50% of cases are diagnosed with a clinical examination alone, so normal physical examination findings cannot exclude CHD. Multiple previous studies have proven Masimo SET pulse oximetry, along with routine clinical examination, is an effective method capable of closing this diagnostic gap to reduce the morbidity and mortality associated with CHD. In contrast, several studies using other pulse oximetry technologies have not shown effectiveness in reliably detecting CHD.
In the study, researchers from three obstetric departments in Mannheim, Germany, measured the postductal oxygen saturation of a total of 3,364 newborns between 6 and 36h of age over a year-long period. Masimo SpO2 measurements were taken via noninvasive sensors placed on the right or left foot (using the VitaGuard VG 310 with integrated Masimo SET technology). The length of the measurement procedure was 2-5min. A standardized protocol was followed: In asymptomatic newborns with SpO2 ≥ 95%, no further steps were taken. For newborns with values 90% - 94%, a check-up assessment was carried out 4-6h later and for persistent SpO2 < 90%, immediate echocardiography was ordered. Following the standardized protocol, researchers found 18 babies that qualified for echocardiographic exam and 9 were diagnosed with CHD. Five of the nine babies had normal auscultation finding in their physical exam, leading researchers to conclude: "These newborns were thus saved from potential cardiogenic shock and even sudden unexpected death." On the basis of this data, a sensitivity of 82% and a specificity of 99.9% with a positive predictive value of 50% and negative predictive value of 99.9% were calculated for the group.
The researchers concluded: "As the method is simple and reliable, has low cost, is not time consuming, creates no additional burden for the patient, and is available and usable anywhere, we would recommend that pulse oximetry screening, with its high sensitivity and specificity levels, should become established as a general screening method in the routine evaluation of the newborn."
Dr. Balaji Govindaswami, Chief of Neonatology and Director of the NICU at Santa Clara Valley Medical Center, stated, "There is overwhelming evidence that screening newborns with Masimo SET pulse oximetry improves our ability to recognize critical congenital heart disease in the first hours of life and that such early detection improves survival. We have implemented pulse oximetry screening of all newborns with very positive results and believe that, based on the evidence and real-world success of CHD screening programs, physicians should advocate this approach as a standard of care in all nurseries."
1 Tautz J, Merkel C, Loersch F, Egen O, Hägele F, Thon HM, Schaible T. "Implication of Pulse Oxymetry Screening for Detection of Congenital Heart Defects." Klinische Pädiatrie May 10, 2010-Epub ahead of print. For further reading, visit: https://www.thieme-connect.com/ejournals/abstract/klinpaed/doi/10.1055/s-0030-1253391.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SET pulse oximetry noninvasive measurements will provide sufficient sensitivity and specificity to help clinicians proactively screen for and detect CHD, and risks related to our belief that Masimo SpO2 measurements and thresholds will be useful in detecting CHD in all newborns, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Fukuda Denshi Signs Technology Licensing Agreement to Integrate Masimo Rainbow SET® Pulse CO-Oximetry™ into 2010 Patient Monitors
One of Japan's Largest Medical Manufacturers Establishes Pulse CO-Oximetry as the Blood Constituent Monitoring Platform of Choice for Next Generation Monitors
Irvine, California – June 1, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, and Fukuda Denshi, a leading global manufacturer of electronic medical devices and patient monitors, today jointly announced a worldwide technology licensing agreement to integrate Masimo Rainbow SET Pulse CO-Oximetry technology into Fukuda Denshi's next generation patient monitors. The agreement allows Fukuda Denshi to incorporate the breakthrough noninvasive blood constituent measurement capabilities of Masimo Rainbow SET to detect and treat life-threatening conditions earlier.
The breakthrough noninvasive blood constituent monitoring capabilities of Masimo Rainbow SET Pulse CO-Oximetry allow for continuous monitoring of hemoglobin, carbon monoxide, methemoglobin, PVI for fluid responsiveness, perfusion index, and measure-through motion pulse oximetry. With Masimo Rainbow SET, the ability to detect internal bleeding, carbon monoxide poisoning, methemoglobinemia, fluid responsiveness, perfusion, saturation, and pulse rate quickly, easily and continuously can help save lives and improve patient outcomes.
According to Kotaro Fukuda, President and CEO, "Our overriding goal is to provide innovative medical devices with superior performance and value. The integration of Masimo Rainbow SET Pulse CO-Oximetry measurements will allow us to design and manufacture state-of-the-art patient monitors that advance the delivery of healthcare. Our combined product offering will allow clinicians to more thoroughly assess a patient's physiological status and make better clinical and treatment decisions."
OEM President, Rick Fishel, stated, "Fukuda Denshi is the first patient monitoring manufacturer in Japan to integrate Masimo Rainbow SET Pulse CO-Oximetry technology. This first-to-market commitment and strategy provides Fukuda Denshi customers with access to an upgradable platform of blood constituent monitoring parameters, leveraging additional clinical intelligence and vital noninvasive blood constituent data on their multiparameter patient monitor."
About Fukuda Denshi
Fukuda Denshi Co. Ltd., is a leading manufacturer of electronic medical devices including a unique set of world-class patient monitoring products. Founded in 1939, Fukuda Denshi is one of Japan's largest medical manufacturers with global offices throughout the world and a network of distributors serving Asia, Europe, North & South America, and Africa. Additional information about Fukuda Denshi and its products may be found at www.fukuda.co.jp
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that Fukuda Denshi's 2010 or future generation patient monitors will feature Masimo Rainbow SET technology; that all Masimo Rainbow SET capabilities will be available in Fukuda Denshi patient monitors; that integration of Masimo Rainbow SET technology and capabilities will allow clinicians using Fukuda Denshi patient monitors to detect internal bleeding, carbon monoxide poisoning, methemoglobinemia, fluid responsiveness, perfusion, saturation, and pulse rate quickly, easily and continuously in all patients; and that using Masimo Rainbow SET will help save lives and improve patient outcomes, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Study Finds Masimo PVI Beneficial in Identifying Patients At-Risk for Developing Anesthesia-Induced Hypotension
Researchers Show Linkage Between Pre-Anesthesia PVI Levels and the Magnitude of Blood Pressure Reduction During Anesthesia Induction
Irvine, California – May 21, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that a new clinical study published in the May 2010 issue of Acta Anaesthesiologica Scandinavica shows that Masimo pleth variability index (PVI) can be used to help clinicians assess risk for mean arterial pressure (MAP) decreases and subsequent hypotension during anesthesia administration. Researchers concluded that PVI may be "useful to identify patients at high risk for developing severe hypotension during anesthesia induction", allowing "anesthesiologists to adopt preventive measures to ensure greater patient safety."1,2
Hypotension (abnormally low blood pressure) during anesthesia induction is a common event that can deprive tissues of adequate oxygen delivery. Severe or sustained hypotension can result in brain, heart, and organ damage—making prompt identification and treatment during the induction period critically important. The ability to properly identify patients at-risk for hypotension could help anesthesiologists to plan preventive strategies to reduce patient risk and ensure safer inductions. Anesthesia-induced hypotension is linked to both patient volume status and vascular tone, and previous studies have shown PVI's association with these physiologic changes.
In the study, researchers from the Osaka City University Medical School in Japan measured PVI (via the Masimo Radical-7 Pulse CO-Oximeter), heart rate (HR), and blood pressure [systolic blood pressure (SBP), diastolic blood pressure (DBP), and MAP] in 76 healthy adult surgical patients at 30-second intervals before and during anesthesia induction (bolus administration of 1.8 mg/kg propofol and 0.6 mg/kg rocuronium). Analysis demonstrated that HR, SBP, DBP, and MAP were significantly decreased after anesthesia administration by 8.5%, 33%, 23%, and 26%, respectively. Pre-anesthesia PVI, which ranged from 7 to 28 with a mean value of 16 +/- 5.5, correlated significantly (r -0.73) with the decrease in MAP. Researchers found that a pre-anesthesia PVI value >15 successfully predicted a decrease in MAP of >25 mmHg with 79% sensitivity, 71% specificity, 73% positive predictive value, and 77% negative predictive value. According to researchers, PVI represents an "easy to perform, noninvasive, and inexpensive method for predicting patients who may develop severe hypotension."
PVI may show changes that reflect physiologic factors such as vascular tone, circulating blood volume, and intrathoracic pressure excursions. PVI is available as part of Masimo Rainbow SET Pulse CO-Oximetry—a breakthrough noninvasive monitoring platform capable of measuring multiple blood constituents that previously required invasive procedures, including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa™), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI).
1 Tsuchiya, M., Yamada, T., and Asada, A. "Pleth Variability Index Predicts Hypotension During Anesthesia Induction." Acta Anaesthesiologica Scandinavica. May 2010, vol. 54, issue 5, pages 596-602. Available online here.
2 This specific utility of PVI has not been reviewed by the US FDA.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo PVI will be useful in predicting anesthesia-induced hypotension in all surgical patients, and risks related to our belief that Masimo Rainbow SET Pulse CO-Oximetry noninvasive measurements (specifically SpHb, PVI, and SpO2) will provide sufficient sensitivity and specificity to help clinicians proactively monitor and manage hemoglobin, fluid and oxygen saturation levels more appropriately and conservatively for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Staten Island University Hospital in New York Adds New Noninvasive Patient Monitoring Capabilities with System-wide Conversion to Masimo Oximetry Technology
Hospital Adds 51 Noninvasive Hemoglobin Monitors to Advance Bloodless Medicine
Irvine, California – May 12, 2010 – Staten Island University Hospital and Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today jointly announced the system-wide conversion of Staten Island University Hospital to Masimo's advanced noninvasive oximetry technology. The conversion ensures that all hospital patients will be noninvasively monitored using the most technologically and clinically-advanced oximetry technology available—providing immediate, real-time measurements that help clinicians more rapidly assess, diagnose, and treat patients.
As part of the conversion, 51 noninvasive hemoglobin monitors have been installed in key care areas throughout the hospital—allowing clinicians to noninvasively measure patient hemoglobin levels in seconds, without the pain and delay associated with traditionally invasive blood tests.
"We wanted access to the most advanced noninvasive oximetry technologies available throughout our entire hospital system and that's exactly what our conversion to Masimo enables," stated Charles T. Vonfrolio, M.D., Director of Anesthesia at Staten Island University Hospital. "Masimo Rainbow SET capabilities provide real-time measurements and new clinical detail that expand patient monitoring beyond the traditional standard of care. With continuous tracking and trending of hemoglobin, fluid status, oxygen, carbon monoxide, and methemoglobin blood levels, we're able to make better healthcare decisions, faster."
The system-wide conversion standardizes all Staten Island University Hospital's sites of care to Masimo technology, including two campuses and 714 hospital beds, which involved upgrading the hospital's multi-parameter patient monitors, pulse oximeters, and sensors. Masimo offers the only upgradable pulse oximetry technology platform—Masimo Rainbow SET Pulse CO-Oximetry—that allows hospitals to add breakthrough noninvasive blood constituent measurement capabilities that previously required invasive procedures. The ability to continuously and noninvasively measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa™), in addition to Masimo SET 'gold standard' Measure-Through Motion and Low Perfusion oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR) simultaneously facilitates earlier detection of life-threatening conditions and helps to guide treatment options.
According to Masimo Executive Vice President of Medical Affairs, Michael O'Reilly, M.D., "Staten Island University Hospital's commitment to leverage state-of-the-art medical technology for the betterment of patient care was a driving force in their decision to upgrade their oximetry technology. Their system-wide conversion to Masimo provides an advanced technology platform offering state-of-the-art noninvasive measurement capabilities they can use today with the ability to easily add new measurements in the future."
About Staten Island University Hospital
Staten Island University Hospital, a member of the North Shore-LIJ Health System, is a 714-bed, specialized teaching hospital located in New York City's 5th and fastest-growing borough. Occupying two large campuses, plus a number of community-based health centers and labs, the hospital provides quality care to the people of Staten Island, the New York metropolitan region, and to patients from around the world. For more information visit www.siuh.edu.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET and Masimo Rainbow SET oximetry technologies will provide sufficient sensitivity and specificity to accurately detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, risks related to our belief that Masimo SET and Rainbow SET measurements will help clinicians guide treatment options, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Arleen Ryback
Staten Island University Hospital
arleen_ryback@siuh.edu
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Study Reveals Startling 28% Increase in Significant Bleeding of Mothers After Delivery
Data Supports Recent Call by Joint Commission to Change Current Practices
Irvine, California – May 6, 2010 – A new study of U.S. maternal outcomes after vaginal and cesarean deliveries published in the May 2010 issue of Anesthesia & Analgesia, a peer-reviewed academic journal, shows that between 1995 and 2004, postpartum hemorrhage (excessive blood loss after delivery) increased 28% in prevalence.1 Postpartum hemorrhage is one of today's most common complications for delivering mothers that according to the study, "markedly increases the odds of in-hospital mortality" and causes 19% of in-hospital maternal deaths. In support of this and other recent data, earlier this year the Joint Commission issued a sentinel event alert calling for protocols and training to improve the ability to detect hemorrhage and other conditions that put mothers at high risk.
In the study, researchers from the Massachusetts General Hospital, Harvard Medical School, and Columbia University College of Physicians and Surgeons used the largest U.S. database of hospitalizations to define and assess trends and risk factors in the incidence of postpartum hemorrhage. Their analysis included discharge datasets from 876,641 hospital admissions for obstetric deliveries in 2004, revealing that postpartum hemorrhage occurred in 25,654 cases, at a rate of 3 per 100 deliveries. Changes in both obstetric practice and maternal demographics in the U.S.—including the increased rate of cesarean delivery, larger proportion of multiple gestation births, and advanced maternal age—may be contributing factors for this deadly trend.
The increase in postpartum hemorrhage was primarily due to an increase in uterine atony—accounting for 79% of all cases. Uterine atony—failure of the uterus to contract maximally after delivery of the baby and placenta—results in heavy uterine (internal) bleeding that can be difficult to identify after delivery (postpartum). Unlike other maternal and fetal risk factors that predispose high-risk deliveries, such as abnormal placentation, which can frequently be identified by predelivery imaging and triaged accordingly, uterine atony does not have identifiable antepartum risk factors and cannot be identified before delivery—making it more difficult to detect and treat. Because of this, patients who hemorrhage from uterine atony (with severity significant enough to require transfusion) represent the greatest challenge to anesthesiologists and obstetricians.
According to researchers: "Because prompt recognition and resuscitation may limit the morbidity and mortality associated with postpartum hemorrhage, 2,3 our results suggest that anesthesiologists practicing in all labor and delivery settings need to have systems in place to manage these patients." Study findings also showed that "postpartum hemorrhage is more common among patients delivering at hospitals in the bottom quartile for delivery volume compared with those delivering at hospitals in the top quartile" and since both anesthesiologists and obstetricians often cover multiple hospitals, researchers cite a need for prudent establishment of "protocols to expedite access to blood products and additional trained personnel."
Based on the increasing risk and growing tragedy of preventable maternal deaths, industry standards and guidelines are also calling for the assessment of key indicators and early warnings signs of hemorrhage during and after delivery. Earlier this year in a Sentinel Event Alert, The Joint Commission warned of this disturbing trend and the increasing rate of maternal mortality.4 Citing the leading cause of maternal death as hemorrhage, the Joint Commission calls out its 2010 standards as a key opportunity for preventing maternal deaths. The Joint Commission Provision of Care, Treatment and Services Standard PC.02.01.19 requires hospitals to: 1) Have a process for recognizing and responding as soon as a patient's condition appears to be worsening; 2) Develop written criteria describing early warning signs of a change or deterioration in a patient's condition and when to seek further assistance; 3) Based on the hospital's early warning criteria, have staff seek additional assistance when they have concerns about a patient's condition; 4) Inform the patient and family how to seek assistance when they have concerns about a patient's condition. Additionally, last year the California Maternal Quality Care Collaborative (CMQCC) released the Obstetric Hemorrhage Care Guidelines Checklist recommending pre-screening, ongoing risk assessment for hemorrhage, and ongoing quantitative evaluation of blood loss on every birth.5
Brian Jones, M.D., Medical Director of OB Anesthesia at Sutter Medical Center in Sacramento and Assistant Professor of Anesthesiology at the University of California-Davis, says that: "This study provides strong evidence of a growing deadly trend—the frequency of serious maternal postpartum hemorrhage is increasing at an alarming rate. Having established protocols in place, such as the CMQCC Obstetric Hemorrhage Care Guidelines, will help physicians and hospitals to have an organized approach for dealing with maternal hemorrhage. And today, new technologies like Masimo noninvasive and continuous hemoglobin (SpHb) offer the opportunity to provide more vigilant and proactive care because it allows clinicians to quickly measure current hemoglobin levels and continuously track them in real-time to detect falling hemoglobin levels that represent an early sign of a potential hemorrhage. With SpHb, clinicians have the ability to detect hemoglobin changes earlier, so they can intervene sooner to help protect delivering mothers from the disastrous consequences of postpartum hemorrhage."
Maternal bleeding is typically discovered after a significant change in vital signs, signs, and/or symptoms, and then confirmed with an invasive laboratory hemoglobin test. This approach can result in late detection of bleeding that can affect patient outcome. Masimo Rainbow SET Pulse CO-Oximeters provide the first-and-only continuous and noninvasive hemoglobin (SpHb) measurements, which can help clinicians quickly identify significant changes hemoglobin levels. When used with other clinical variables, Masimo SpHb may help clinicians assess anemic status and help determine treatment and additional test options.
Madhava Karunarathna, MD, an OB/GYN at Balangoda Hospital in Sri Lanka, stated: "In cases of severe hemorrhaging during and after childbirth, SpHb has enabled us to immediately identify and continuously assess blood loss severity to better manage internal bleeding, prevent overloading of fluid, and decrease maternal death."
Masimo Rainbow SET Pulse CO-Oximetry is a breakthrough noninvasive blood constituent monitoring platform measuring multiple blood constituents that previously required invasive procedures, including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, acoustic respiration rate (RRa™), oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI). Masimo SpHb, PVI, and SpO2 have been shown in multiple clinical studies to provide accurate, reliable, real-time measurements that help clinicians to proactively monitor and manage hemoglobin, fluid, and oxygen saturation levels.
1 Bateman BT, Berman MF, Riley LE, Leffert LR." The Epidemiology of Postpartum Hemorrhage in a Large, Nationwide Sample of Deliveries" Anesth Analg May 2010 110:1368-1373. For further reading, visit: http://www.anesthesia-analgesia.org/content/110/5/1368.abstract.
2 Mercier FJ, Van de Velde M. "Major Obstetric Hemorrhage." Anesthesiol Clin 2008;26:53–66.
3 Mahutte NG, Murphy-Kaulbeck L, Le Q, Solomon J, Benjamin A, Boyd ME. "Obstetric Admissions to the Intensive Care Unit." Obstet Gynecol 1999;94:263–6.
4 The Joint Commission, "Sentinel Event Alert: Preventing Maternal Death" Issue 44, January 26, 2010. For further reading, visit: http://www.jointcommission.org/sentinel_event_alert_issue_44_preventing_maternal_death/.
5 California Maternal Quality Care Collaborative (CMQCC) "Obstetric Hemorrhage Care Guidelines Checklist." For further reading, visit: http://www.cmqcc.org/resources/ob_hemorrhage/ob_hemorrhage_protocol_tools_version_1_2.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to risks related to our beliefs that Masimo Pulse CO-Oximeters provide the first-and-only commercially available and clinically-proven monitoring solutions for the ongoing, continuous, noninvasive measurement of hemoglobin levels; Masimo SpHb will help clinicians to immediately detect anemia, identify internal bleeding earlier, and maintain tighter transfusion management protocols and controls; and Masimo Rainbow SET Pulse CO-Oximetry noninvasive measurements (specifically SpHb, PVI, and SpO2) will provide sufficient sensitivity and specificity to help clinicians proactively monitor and manage hemoglobin, fluid and oxygen saturation levels more appropriately and conservatively for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Masimo Corporation Media Contact:
Dana Banks
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports First Quarter 2010 Financial Results
Q1 2010 Highlights:
- Total revenues rose 15.5% to $98.8 million, compared to the first quarter 2009
- Product revenues rose 15.3% to $85.9 million, compared to the first quarter 2009
- Shipments of Masimo SET and Masimo Rainbow SET units rose 34% to 37,100 units, compared to the first quarter 2009
- Rainbow revenues rose 71.3% to $5.3 million, compared to the first quarter 2009
- GAAP EPS of $0.44 included a $0.20 net gain related to antitrust lawsuit victory. Adjusted EPS of $0.24, compared to $0.22 in the first quarter of 2009
Irvine, California, May 4, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, today announced its financial results for the first quarter of 2010.
Masimo's total revenues for the first quarter were $98.8 million, up 15.5% from $85.5 million for the first quarter of 2009. Masimo's first quarter product revenues rose 15.3% to $85.9 million, compared to $74.5 million for the first quarter of 2009. Revenues from Masimo Rainbow SET products rose 71.3% to $5.3 million in the first quarter, compared to $3.1 million for the first quarter of 2009.
Net income for the first quarter was $26.7 million, or $0.44 per diluted share, including the after-tax impact of $30.1 million in proceeds from the antitrust lawsuit victory against Covidien, partially offset by $10.9 million in related, incremental one-time SG&A expenses. Excluding this net one-time pre-tax gain of $19.2 million, net income for the first quarter was $14.3 million, or $0.24 per diluted share, compared to net income of $13.0 million, or $0.22 per diluted share, in the first quarter of 2009.
During the first quarter, the company shipped 37,100 Masimo SET pulse oximetry and Masimo Rainbow SET Pulse CO-Oximetry units, excluding handheld units, compared to 27,700 in the same period last year. Masimo estimates its worldwide installed base as of April 3, 2010 to be approximately 757,000 units, up 16% from approximately 651,000 for the same period last year.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Masimo's mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and new applications. Toward this mission, we made solid progress through double-digit growth in our core Masimo SET pulse oximetry and a 71% increase in sales of Masimo Rainbow Pulse CO-Oximetry, which includes the first-ever continuous and noninvasive hemoglobin measurement. The 34% year-over-year growth in unit shipments of Masimo SET and Masimo Rainbow monitors and OEM boards reflects strong sales to OEMs and growing demand for both our Rainbow platform, and Patient SafetyNet system for the general floor."
As of April 3, 2010, cash, cash equivalents and short-term investments totaled $112.1 million, compared to $189.0 million as of January 2, 2010. The decline is due to the March 31, 2010 dividend payment of $117.5 million, partially offset by operating cash flow and the net proceeds from the antitrust lawsuit.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation code for both dial-in numbers is 69324525. A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. After the live webcast, the call will be available on Masimo's website through June 4, 2010. In addition, a telephonic replay of the call will be available through May 18, 2010. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 69324525.
About Masimo
Masimo (Nasdaq: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo Rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any new products and technologies of ours; obtaining regulatory approval of our current and future products and technologies, including the recently announced total hemoglobin measurement; the risk that the implementation of our international realignment, will not produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Masimo Corporation
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
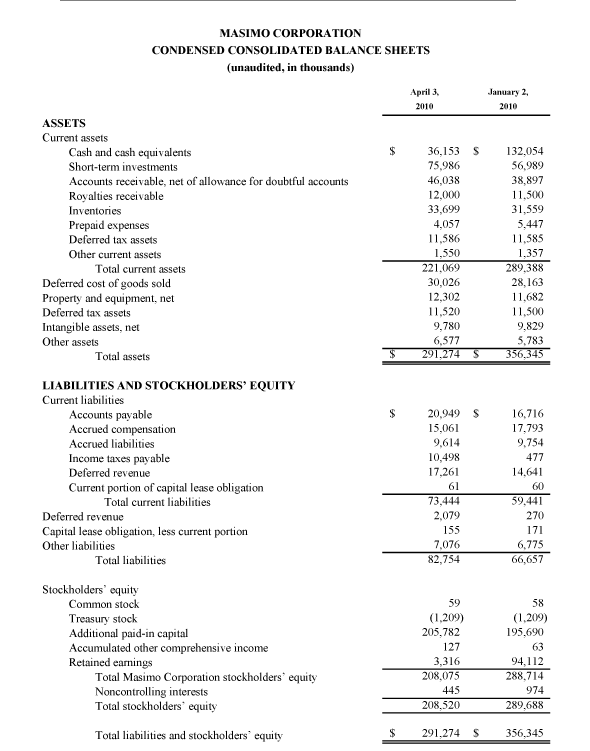
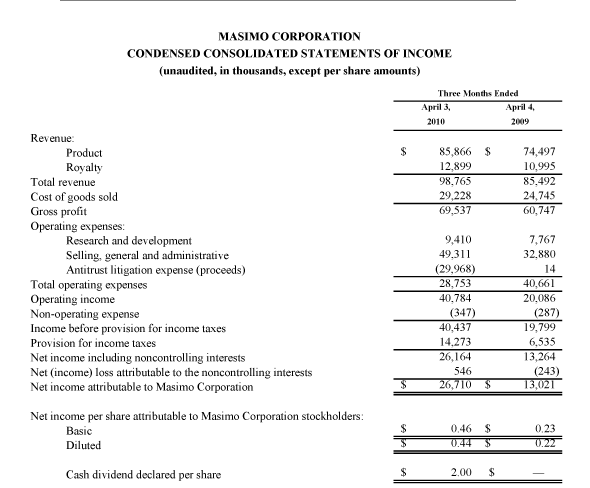
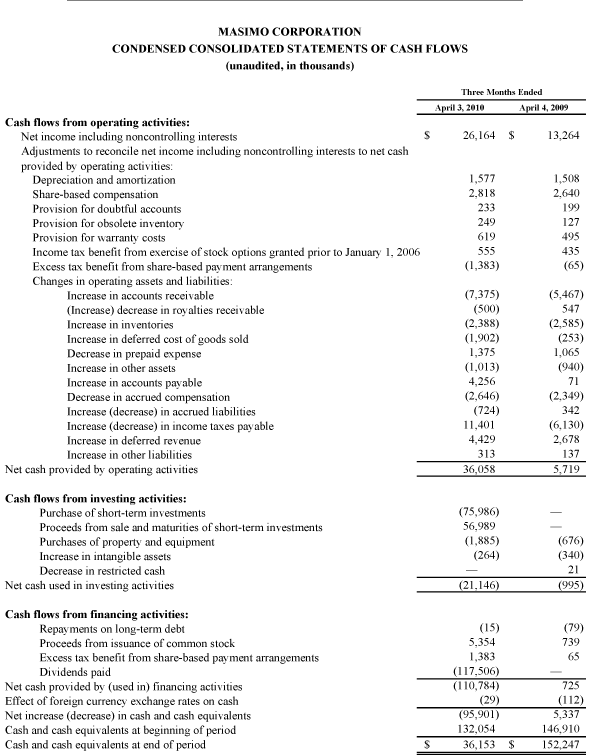


COUNTY OF LOS ANGELES FIRE MUSEUM PRESENTS "PIONEERS OF PARAMEDICINE™" LIFETIME ACHIEVEMENT AWARD
The four founding fathers of paramedic programs are honored at the inaugural induction ceremony and awards gala on May 8, 2010 at the Historic Millennium Biltmore Hotel in Los Angeles, Calif.
Los Angeles, Calif., USA – The County of Los Angeles Fire Museum announced today the induction of Eugene Nagel, MD, Leonard Cobb, MD, J. Michael Criley, MD, and Walter Graf, MD, the four founding fathers of paramedic programs, into the "Pioneers of Paramedicine" exhibit and receipt of the "Pioneers of Paramedicine" Lifetime Achievement Award made possible by founding sponsors, Philips Healthcare and Masimo Corporation.
The "Pioneers of Paramedicine" is a national program created by the County of Los Angeles Fire Museum Association Board of Directors to recognize and honor individuals whose lives and accomplishments exemplify the courage, independence and spirit of innovation that helped shape the development of modern emergency medical services in the United States in the 1960s and 1970s. In addition to annual recognitions, the project will include a video interview series and a permanent exhibit at the museum's future home.
"If not for these determined visionaries who saw that the future of emergency medicine lay outside the hallowed walls of the hospital, the idea of paramedics and EMTs saving lives in the field may not have gotten off the ground for another decade. Countless lives would have been needlessly lost. Americans, and the world, owe them a debt of gratitude for their foresight and perseverance," said Randolph Mantooth, the museum's honorary chairman. "I extend my personal invitation to everyone in the business of saving lives to join us on May 8 for a once in a lifetime opportunity to pay tribute to the men who are not only responsible for the careers you enjoy, but who are truly the 'Pioneers of Paramedicine.'"
The development of the paramedicine industry created a niche of device innovation to support emergency professionals in the field. Only a decade ago, many of the lifesaving devices that emergency responders rely on today were only available in hospitals.
According to Masimo Founder and CEO, Joe Kiani, "As a manufacturer of lifesaving medical monitoring devices, arming emergency first responders with the innovative tools they need to save and preserve life has been a top priority. Today, through the Pioneers of Paramedicine, we honor the heroes who have dedicated their lives to saving all of ours. We proudly support and salute the founding fathers of paramedicine."
"Philips is committed to developing advanced resuscitation products that enable emergency medical professionals to perform lifesaving tasks every day," said Mike Miller, senior vice president and general manager, Cardiac Care for Philips Healthcare. "We are honored to contribute to the recognition of this important discipline and the people who paved the way."
The Gala and awards ceremony will be hosted on Saturday, May 8, 2010 at the historic Millennium Biltmore Hotel in Los Angeles, California. For additional details about the gala, visit http://www.pioneersofparamedicine.org/gala.html. For tickets, email PioneersofParamedicine@EventsbyOne.com.
For media inquiries, please contact:
Nancy McFarland
Nancy@PioneersOfParamedicine.org
ABOUT THE COUNTY OF LOS ANGELES FIRE MUSEUM
The County of Los Angeles Fire Museum Association is a California Public Benefit non profit 501(c)(3) Corporation, established in 1975 and incorporated in 1989. Acquisitions, restorations and operating funds are supported by monthly and annual membership dues paid by 3,000 active duty Los Angeles County Firefighters, retirees, members of other fire departments, and the public. The museum's collection includes over sixty historical apparatus, some dating back to the late 1800s, as well as hundreds of artifacts and thousands of photographs. The most popular vehicles in the collection are the original paramedic/rescue Squad 51 and Engine 51 from the 1970s television show EMERGENCY! The collection is currently housed in warehouse locations in South Gate and Bellflower, California. The Museum Association is governed by a seven-member board of directors, nominated and elected by the membership every two years. James O. Page was the sitting president at the time of his passing in 2004. Randolph Mantooth has served as honorary chairman and spokesperson since 2005. Updates on current activities, operating hours, and plans for future construction are found at www.LACountyFireMuseum.com.
About Royal Philips Electronics
Royal Philips Electronics of the Netherlands (NYSE: PHG, AEX: PHI) is a diversified Health and Well-being company, focused on improving people's lives through timely innovations. As a world leader in healthcare, lifestyle and lighting, Philips integrates technologies and design into people-centric solutions, based on fundamental customer insights and the brand promise of "sense and simplicity". Headquartered in the Netherlands, Philips employs more than 116,000 employees in more than 60 countries worldwide. With sales of US$32.3 billion in 2009, the company is a market leader in cardiac care, acute care and home healthcare, energy efficient lighting solutions and new lighting applications, as well as lifestyle products for personal well-being and pleasure with strong leadership positions in flat TV, male shaving and grooming, portable entertainment and oral healthcare. News from Philips is located at www.philips.com/newscenter.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.


Masimo to Present at Bank of America Merrill Lynch Health Care Conference
IRVINE, Calif., April 29, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that its management is scheduled to present at the Bank of America Merrill Lynch Health Care Conference at The Grand Hyatt in New York on Wednesday, May 12, at 3:40 p.m. ET. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET® provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Establishes Non-Profit Foundation to Promote Ethics, Innovation, and Competition in Healthcare
Global Medical Technology Innovator Provides Initial $10 Million Monetary Gift and Contribution of Masimo SET and Masimo Rainbow SET Oximetry Equipment and Supplies
Irvine, California – April 28, 2010 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today the formation of The Masimo Foundation for Ethics, Innovation and Competition in Healthcare, a non-profit organization that will facilitate its corporate philanthropy. During the first quarter of 2010, Masimo provided a monetary gift of $10 million and an in-kind contribution of pulse oximetry equipment and supplies to support the foundation's efforts.
Masimo Founder and Chief Executive Officer Joe Kiani will serve as Chairman of The Masimo Foundation. The Foundation's purpose will be to encourage and promote various activities, programs, and research opportunities designed to improve patient safety and deliver advanced health care to people worldwide who may not otherwise have access to lifesaving technologies. In addition, the Foundation intends to support third-party research, development initiatives, and clinical studies designed to expand the healthcare industry's ability to provide better and more cost-effective solutions and protocols for healthcare delivery throughout the world. Finally, the Foundation intends to provide special attention to those causes whose ultimate goals are ethical—focused on doing the right thing for the right reasons—and designed to create healthy competition, which we believe is the ultimate answer to lower health care costs in the U.S. and throughout the world.
Mr. Kiani stated, "Masimo has long been dedicated to its mission to improve patient outcomes and reduce the cost of care through innovations in noninvasive monitoring. Similarly, The Masimo Foundation will dedicate itself to improving patient care through philanthropic programs and research initiatives that foster an environment of robust and honest competition, and enhance caregiver access to cost-effective and innovative healthcare solutions."
Masimo's $10 million gift to The Masimo Foundation represents a portion of the $30 million payment it received in January 2010, following the Ninth Circuit Court of Appeals' October 2009 affirmance of a Federal District Court judgment in Masimo's favor that Tyco Healthcare, now Covidien, violated the antitrust laws through anticompetitive business practices related to the sale of its pulse oximetry products.
Currently, the Foundation is in the process of formalizing its grant-making guidelines and will be developing a comprehensive website that will serve as a resource for those interested in learning more about specific programs and grant opportunities aimed at fostering ethics, innovation and competition in healthcare. In the meantime, letters of inquiry may be sent to Ann L. Van Dormolen, Administrator, Masimo Foundation, P.O. Box 9399, Marina del Rey, CA, 90295.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to benefits of one-time spending associated with the recovery related to the antitrust matter, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Masimo Foundation Contact:
Ann L. Van Dormolen, Administrator
P.O. Box 9399
Marina del Ray, CA 90295
Masimo Corporation Media Contact:
Dana Banks
(949) 297-7348
dbanks@masimo.com
Masimo Corporation Investor Contact:
Sheree Aronson
(949) 297-7043
saronson@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report First Quarter 2010 Financial Results after Market Close on May 4, 2010
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., April 21, 2010 -- Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced it will release first quarter 2010 financial results for the period ended April 3, 2010, after the market closes on May 4, 2010.
A conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe E. Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation code for both dial-in numbers is 69324525. After the live webcast, the call will be available on Masimo's website through June 4, 2010. In addition, a telephonic replay of the call will be available through May 18, 2010. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 69324525.
About MasimoMasimo (Nasdaq: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRaTM). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Nassau University Medical Center in New York Adds Noninvasive Patient Monitoring Capabilities with Masimo Pulse Oximetry & Pulse CO-Oximetry™ Conversion
New Noninvasive Medical Technology Advancements Improve Patient Monitoring, Safety, and Quality
Irvine, California – April 14, 2010 – Nassau University Medical Center (NUMC) and Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today jointly announced completion of NUMC's system-wide conversion to Masimo oximetry technologies. The conversion ensures that NUMC patients will be monitored noninvasively using the most technologically and clinically-advanced pulse oximetry and Pulse CO-Oximetry technologies available—providing real-time results for vital measurements that help clinicians to more rapidly assess, diagnose, and treat patients.
"Converting to Masimo has greatly advanced our noninvasive patient monitoring capabilities and we've been particularly happy with the time it saves us during critical trauma cases," said Paul Weinberg, M.D., Chairman of Anesthesia at Nassau University Medical Center. "With Masimo noninvasive hemoglobin, we see the trend much quicker so we know where the patient's blood volume is heading and can better anticipate what needs to be done. It's unique to have immediate access to this level of clinical detail with the ability to track blood levels so accurately."
The system-wide conversion standardizes all of NUMC's sites of care, including over 530 hospital beds, to Masimo technology, which involved upgrading the hospital's multiparameter patient monitors, pulse oximeters, and sensors. Masimo offers the only upgradable pulse oximetry technology platform—Masimo Rainbow SET Pulse CO-Oximetry—that allows hospitals to add breakthrough noninvasive blood constituent measurement capabilities that previously required invasive procedures. The ability to continuously and noninvasively measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa™), in addition to Masimo SET 'gold standard' Measure-Through Motion and Low Perfusion oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR) measurements enables monitoring of multiple physiological parameters simultaneously—to help clinicians detect life-threatening conditions and help guide treatment options.
Masimo Founder and CEO, Joe E. Kiani, said, "Nassau University Medical Center's conversion to Masimo's oximetry technology provides clinicians with the patient monitoring tools they need to increase early detection of physiological deterioration and improve safety. The noninvasive and continuous measurements provided by Masimo Rainbow SET Pulse CO-Oximetry arms clinicians with new visibility into the physiology and underlying condition of their patients—helping to expand their clinical capabilities."
About the NuHealth System
NuHealth is a Long Island health care organization delivering essential medical care and disease and lifestyle management to everyone at every stage of life. Also known as Nassau Health Care Corporation, NuHealth is a public benefit corporation managing the operations of Nassau University Medical Center, A. Holly Patterson Extended Care and a network of Family Health Centers that bring primary and specialty care out into the community. By emphasizing wellness, cultural sensitivity and collaborative efforts with the North Shore-LIJ Health System, NuHealth is working to make good care more affordable and easier to access. For more information about NuHealth or its Centers of Care, visit www.nuhealth.net.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET and Masimo Rainbow SET oximetry technologies will provide sufficient sensitivity and specificity to accurately detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, risks related to our belief that Masimo SET and Rainbow SET measurements will help clinicians guide treatment options, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Shelley Lotenberg
Nassau University Medical Center
(516) 572-6055
Shelley@numc.edu
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Published Study Finds the Cost of Blood Transfusions is Significantly Under-Estimated, Establishes True Cost at $522 to $1,183 Per Unit
Annual Costs Total $1.6 to $6 Million Per Hospital Surveyed—Warranting Improved Conservation & Cost Containment Strategies
Irvine, California – April 5, 2010 – A new blood transfusion cost analysis study published in the April 2010 issue of Transfusion, a peer-reviewed academic journal, shows that when all of the complex cost factors leading up to and after a red blood cell (RBC) transfusion are considered, the actual cost of blood is substantially higher than previously estimated. With actual blood transfusion costs ranging between $522 and $1,183 per-unit—37% higher than estimated by prior studies, which did not include all associated costs—the new study calculates that the true cost of blood is 3.2 to 4.8-fold higher than reported blood product acquisition costs.1
"Representing the most detailed and rigorous method utilized to date to account for the cost of blood transfusions," study findings confirm that annual expenditures on blood and transfusion-related activities for surgical patients are significant resource drains—costing between $1.6 to $6.0 million per hospital surveyed.
In the study, researchers from the Society for the Advancement of Blood Management (SABM) and the Medical Society for Blood Management (MSBM) prospectively analyzed 20,104 surgical patients who had their blood typed and screened in preparation for a blood transfusion at two U.S. and two European hospitals. After precisely mapping all diagnostic, therapeutic, technical, laboratory, logistic, administrative, informational, educational, and quality activities involved in the transfusion of blood in real-world surgical settings, researchers constructed an activity-based cost model capturing all the actual direct and indirect costs of acquiring, delivering, administering, and monitoring RBC transfusions from the hospital perspective—yielding "for the first time a dollar amount for the cost per unit of blood that reflects the complexities of real-world blood utilization."
Study findings also showed that "total annual blood costs are largely driven by transfusion rate," which includes factors such as the proportion of surgical patients transfused and the number of RBC units per patient transfused, and provide a unique understanding of both cost drivers and the opportunities for cost containment. According to researchers, "reducing either or both factors has the potential to reduce costs dramatically."
Most importantly, the study's activity-based cost model provides a "roadmap for institutional administrators worldwide to evaluate hospital processes and the impetus to initiate programs to reduce and optimize blood usage," says lead researcher and the President-elect of SABM, Aryeh Shander, M.D., who is also the Executive Medical Director for The Institute for Patient Blood Management & Bloodless Medicine and Surgery at Englewood Hospital and Medical Center in Englewood, New Jersey. Dr. Shander believes that this study spotlights the incredibly complex resource and cost drains associated with real-world blood transfusions, offering hospitals and healthcare providers an important cost saving insight that "improved blood testing techniques and blood conservation strategies provide unique opportunities to significantly reduce the number of unnecessary blood transfusions and the quantity of units administered—delivering better cost containment and patient benefits."
While multiple studies have shown that blood transfusions increase morbidity and mortality, the present study did not attempt to evaluate the morbidity-associated costs of blood transfusions. Thus, the cost estimate presented in this study may still underestimate the cost of giving blood transfusions.
Masimo Rainbow SET Pulse CO-Oximetry—a breakthrough noninvasive blood constituent monitoring platform measuring multiple blood constituents that previously required invasive procedures, including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, acoustic respiration rate (RRa™), oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI). Masimo SpHb, PVI, and SpO2 have been shown in multiple clinical studies to provide accurate, reliable, real-time measurements that help clinicians to proactively monitor and manage hemoglobin, fluid, and oxygen saturation levels more appropriately and conservatively.
1 Shander, A., Hofmann, A., Ozawa, S., Theusinger, O., Gombotz, H., Spahn, D. "Activity-based Costs of Blood Transfusions in Surgical Patients at Four Hospitals." Transfusion. April 2010, Volume 50, Issue 4, Pages 753-765. For further reading, visit: http://www3.interscience.wiley.com/journal/123208754/abstract.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™ allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Rainbow SET Pulse CO-Oximetry noninvasive measurements (specifically SpHb, PVI, and SpO2) will provide sufficient sensitivity and specificity to help clinicians proactively monitor and manage hemoglobin, fluid and oxygen saturation levels more appropriately and conservatively for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


John Hunter Children's Hospital Installs Australia's First Masimo Patient SafetyNet™ System
Irvine, California – April 1, 2010– Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry announced today the installation of Australia's first Masimo Patient SafetyNet System at John Hunter Children's Hospital in New South Wales. The new, state-of-the-art remote monitoring and wireless clinician notification system is currently being used to noninvasively and continuously monitor the physiological status of selected pediatric patients in the medical ward—ensuring early detection of deterioration or distress and enabling potentially life-saving interventions.
Earlier this week, guests attending the hospital's unveiling saw live demonstrations of the Masimo Patient SafetyNet system in action, including: how a patient is entered into the system and continuously, remotely monitored using Masimo SET® pulse oximeters via a noninvasive finger sensor; how alarms and clinician assignments are set; and how patient data and alarms are wirelessly transmitted from the bedside monitors to the central monitoring station where clinicians are immediately alerted to the patient's deteriorating health condition and can initiate prompt, appropriate rescue and treatment.
(Click here to see the Patient SafetyNet system in action.)

Nursing Unit Manager Cathy Grahame, responding to a Patient SafetyNet Alert, Checks the Masimo oximetry monitor to confirm the patient's condition.
The Patient SafetyNet system keeps patients safer by continuously, noninvasively, and remotely monitoring multiple physiological parameters, including the amount of oxygen in the blood and pulse rate, and automatically alerting clinicians to changes that signal patient distress or deterioration. Results of a landmark two-year clinical study published in February demonstrated that Patient SafetyNet helps to reduce rescue events and activations by 65% and ICU transfers by 48%—contributing to significant improvements in patient outcomes.1
Medical Ward Nursing Unit Manager Cathy Grahame, acknowledging the importance of having such vital equipment, stated, "The Patient SafetyNet system allows us to respond immediately to a patient whose vital signs have changed, which, in this time-critical environment, improves our quality of care."
Masimo Patient SafetyNet is fully-integrated into John Hunter Children's Hospital's existing IT infrastructure with a central monitoring station located at the nurse's station. The central monitoring station displays every Patient SafetyNet monitor on the ward, enabling nursing staff to view vital signs and alarm activations for each patient. Community support, in particular the fundraising efforts of the employees and management of John Holland Pty Ltd, provided the initial funds to purchase the Patient SafetyNet system and 18 monitors. The hospital is hoping to raise funds for more units.
Combining Masimo SET pulse oximetry with respiration rate monitoring at the point-of-care and automated, wireless clinician notifications via pager, Patient SafetyNet provides an unmatched level of safety for up to 80 patients on four floors. The system uses open IEEE industry standards for connectivity, which allows for more efficient sharing of data across a hospital's IT platforms, along with the option of full integration into a hospital's existing IT infrastructure—providing a lower overall cost of ownership and improved financial benefits.

The Director of John Hunter Children's Hospital, Pat Marks (far left), thanks John Holland Pty Ltd. representatives, whose fundraising efforts contributed to the Patient SafetyNet purchase for the medical ward.
1 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading, visit: http://journals.lww.com/anesthesiology/Fulltext/2010/02000/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.10.aspx .
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™ allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of study results, risks related to our assumptions that Masimo Patient SafetyNet will provide an effective early warning system of a patient's deteriorating physiological condition to enable timely rescue, risks related to our belief that Masimo SET pulse oximetry and Masimo Rainbow SET Pulse CO-Oximetry measurements will provide sufficient sensitivity and specificity to detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, and risks related to our assumptions regarding the systems' ability to deliver clinical improvement over alternative patient monitoring and assessment methods to increase patient safety and allow for further adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Multi-Center Study Demonstrates Masimo PVI Predicts the Hemodynamic Effects of Positive End-Expiratory Pressure (PEEP)
Researchers Conclude that PVI May be Useful in Automatically and Noninvasively Detecting the Deleterious Effects of PEEP in Ventilated and Sedated Patients
Irvine, California – March 31, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that a new clinical study published in the March 2010 issue of Anesthesia & Analgesia, shows Masimo PVI successfully predicts the hemodynamic effects of Positive End-Expiratory Pressure (PEEP) in mechanically ventilated patients after cardiac surgery. According to study researchers, the ability of PVI to predict the effects of PEEP may allow physicians to "optimize the respiratory uptake in oxygen and its delivery to the tissues."1
It is critically important for clinicians to accurately determine whether the addition of PEEP, a ventilator setting that can alter cardiac output (the amount of blood the heart pumps), will have positive or negative hemodynamic effects. PEEP can be beneficial if it improves PaO2 (arterial oxygenation and oxygen delivery during anesthesia) by opening collapsed alveoli to increase gas exchange, but harmful if it decreases blood flow to the tissues (most often measured as cardiac output). Masimo PVI has been shown on multiple clinical studies to continuously and noninvasively predict fluid responsiveness in mechanically-ventilated patients under general anesthesia. However, researchers in this study approached the relationship between PVI and fluid responsiveness in the reverse manner from which it is usually evaluated. Instead of looking at whether PVI predicts a positive response by the patient, they evaluated whether PVI predicated a negative response (decreased preload) with the addition of PEEP.
Researchers from the Louis Pradel Hospital, Department of Anesthesiology and Intensive Care, in Lyon, France, and the University of California, Irvine, School of Medicine in Irvine, California, studied 21 mechanically-ventilated and sedated patients in the postoperative period after coronary artery bypass graft (CABG) surgery. Patients were monitored via invasive pulmonary artery catheter for end-expiratory central venous pressure (CVP), end-expiratory pulmonary capillary wedge pressure (PCWP), cardiac index (cardiac output indexed to body surface area) (CI), pulse pressure variation (∆PP), and stroke volume (SV), and PVI via a Masimo Rainbow SET Pulse CO-Oximeter sensor attached to the finger. Hemodynamic data was recorded at three successive tidal volumes (VT of 6, 8, and 10 mL/kg) during zero end-expiratory pressure (ZEEP) and after the addition of 10 cm H2O PEEP for each VT and hemodynamically instable (HI) patients were defined as those with >15% decrease in CI after the addition of PEEP.
Results showed that at a VT of 8 mL/kg, PVI was significantly higher in the HI group than in the non-HI group (13% ± 5% vs 9% ± 4%, P<0.03) and a PVI of 12% at ZEEP predicted a significant decrease in CI after the addition of PEEP in 6 patients with a sensitivity of 83% and a specificity of 80%. ∆PP was also significantly higher in the HI group than in the non-HI group; however CI, CVP, and PCWP were not different. At VT of 10 mL/kg, PVI was still significantly higher in the HI group than in the non-HI group (16% ± 7% vs 10% ± 4%, P<0.01) and a PVI of 13% at ZEEP predicted a significant decrease in CI after the addition of PEEP in 9 patients with a sensitivity of 78% and a specificity of 83%. Researchers concluded that Masimo PVI may be "useful in automatically and noninvasively detecting the hemodynamic effects of PEEP when VT is ≥ 8 mL/kg in ventilated and sedated patients with acceptable sensitivity and specificity."
The current study reinforces the value of PVI compared to invasive measures and highlights PVI's value for reliably detecting the hemodynamic effects of PEEP and predicting hemodynamic instability. PVI is available as part of Masimo Rainbow SET Pulse CO-Oximetry—a breakthrough noninvasive blood constituent monitoring platform capable of measuring multiple blood constituents that previously required invasive procedures, including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI).
1 Desebbe, Olivier, Boucau, Cecile, Farhat, Fadi, Bastien, Olivier, Lehot, Jean-Jacques, and Cannesson, Maxime. "The Ability of Pleth Variability Index to Predict the Hemodynamic Effects of Positive End-Expiratory Pressure in Mechanically-Ventilated Patients Under General Anesthesia." Anesthesia & Analgesia. March 2010, vol. 110, no. 3, pages 792-798. Available online at: www.anesthesia-analgesia.org/content/110/3/792.full.pdf+html
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo PVI will be useful in automatically and noninvasively detecting the deleterious effects of PEEP in all ventilated and sedated patients, and risks related to our belief that Masimo Rainbow SET Pulse CO-Oximetry noninvasive measurements (specifically SpHb, PVI, and SpO2) will provide sufficient sensitivity and specificity to help clinicians proactively monitor and manage hemoglobin, fluid and oxygen saturation levels more appropriately and conservatively for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Oridion and Masimo Sign Distribution Agreement to Sell Capnostream™20 Bedside Monitors with the Masimo Patient SafetyNet™ System
Masimo Patient SafetyNet Remote Monitoring and Clinician Notification System is First to Combine Distribution and Full-Featured Connectivity with Oridion Capnostream™20 Monitors
Jerusalem, Israel/Irvine, California – March 25, 2010 – Oridion (SIX Swiss Exchange: ORIDN), creator of Smart Capnography™, and Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today jointly announced a distribution agreement naming Masimo as a non-exclusive distributor for the Oridion Capnostream™20 portable bedside capnography monitors throughout the U.S., Canada, and Europe.
This distribution agreement further enhances last year's agreement between Masimo and Oridion which established connectivity between the Capnostream™20 bedside monitors and the Masimo Patient SafetyNet™ remote monitoring and clinician notification system. By making the Capnostream™20 available as part of Masimo's general floor monitoring solutions, Masimo Patient SafetyNet is the first remote monitoring and clinician notification system to feature Oridion's newest Smart Capnography™ innovation—the Integrated Pulmonary Index™ (IPI)—enabling real-time tracking and trending of Oridion etCO2 and respiration rate in combination with Masimo SET® SpO2 and pulse rate, for a comprehensive assessment of the patient's oxygenation and ventilatory status.
Gerry Feldman, President of Oridion Capnography Inc. remarks, "We are delighted to partner with Masimo to add the distribution of our Capnostream™20 monitors in conjunction with Masimo's innovative Patient SafetyNet System. This powerful solution combines proven best-in-class performance and measurements for monitoring oxygenation and ventilation in an easy-to-use device for both hospital and outpatient environments, and offers the added safety associated with remote monitoring and automated clinician notification of adverse changes in a patient's status. Cooperation between our two industry-leading companies will result in optimal monitoring options and the industry's most advanced surveillance solutions for healthcare facilities seeking to improve patient safety and clinical outcomes."
The enhanced partnership between the two gold-standard technologies now enables full distribution capabilities in addition to the connectivity and two-way wireless communication announced between Oridion Capnostream™20 and Masimo's Patient SafetyNet System in August 2009. This allows hospitals to purchase and implement these two complementary devices directly from Masimo to obtain the highest level of patient care with the industry's most advanced continuous monitoring and patient surveillance solution.
Oridion Microstream® capnography combines the healthcare industry's most accurate and reliable sidestream etCO2/respiration rate measurements with Oridion Smart Capnography™, which includes SARA™ alarm management and the Integrated Pulmonary Index™ (IPI). IPI integrates Capnography and Pulse Oximetry values into a single, easy to understand index simplifying assessment of patient ventilation status. Masimo SET® provides the most accurate and reliable pulse oximetry technology, clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 and pulse rate measurements and alarms. Masimo SET Pulse Oximetry and Oridion Smart Capnography provide superior algorithm technologies that reduce alarms, improve clinical workflow, and provide valuable decision-support tools to patient caregivers.
According to Masimo Founder and CEO, Joe E. Kiani, "We are proud to deliver upon our promise to clinicians and the industry by expanding the availability of vital sign parameters and third-party multi-parameter patient monitoring devices that offer real-time wireless connectivity to Masimo Patient SafteyNet. This distribution agreement allows us to package two proven, best-in-class solutions, Oridion Capnostream™20 monitors with Masimo SET and Masimo Patient SafetyNet, for hospitals and healthcare facilities in the U.S., Canada, and Europe—offering the flexibility and device options that today's healthcare providers need to unite their patient monitoring solutions."
About Oridion
Oridion Systems Ltd. (www.oridion.com) is a global medical device company specializing in patient safety monitoring. The Company operates through wholly owned subsidiaries in Israel and the United States. Oridion develops proprietary medical devices and patient interfaces, based on its patented Microstream® and Smart Capnography™ technologies, for the enhancement of patient safety and clinical outcomes. These products provide effective, proven airway management and are used in diverse clinical environments, including procedural sedation, pain management, critical care, post-anesthesia care, emergency medical services, transport, alternate care and other settings where patients' respiratory systems may be compromised and at risk. The Oridion commitment to excellence is modeled on making the extraordinary, ordinary and making respiratory monitoring 'Smart' for the patient and the healthcare professional. Additional information about Oridion and its products may be found at www.oridion.com.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the timing and availability of connectivity and a two-way interface between Oridion Capnostream and Masimo Patient SafetyNet, risks related to our belief that Patient SafetyNet will provide an effective early warning system to enable timely rescue, risks related to our belief that Masimo SET will provide sufficient sensitivity and specificity to detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, as well as other factors discussed in the "Risk Factors" section of the Masimo Annual Quarterly Report on Form 10-Q for the fiscal quarter year ended July 4, 2009, filed with the Securities and Exchange Commission ("SEC") on August 6, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. Neither Masimo nor Oridion do undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in the Masimo Quarterly Report on Form 10-Q for the fiscal quarter ended July 4, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Walter Tabachnik
Oridion Systems Ltd.
+972 (2) 589-9159
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. Capnostream and Microstream are trademarks or registered trademarks of Oridion.


New Clinical Study Shows Masimo Rainbow SET® Pulse CO-Oximetry™ Provides Accurate Noninvasive Measurements of Hemoglobin in Pediatric Patients
Study Presented at the International Anesthesia Research Society (IARS) Annual Meeting Also Demonstrates Masimo SpHb™ Provides Earlier Indications of Directional Changes than
Intermittent Invasive Hemoglobin Values
Irvine, California – March 24, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that a new clinical study presented this week at the IARS Annual Meeting in Honolulu, Hawaii, demonstrates that noninvasive and continuous hemoglobin (SpHb) from Masimo Rainbow SET Pulse CO-Oximetry provides comparable accuracy as point-of-care invasive measurements of total hemoglobin versus standard laboratory invasive measurements of total hemoglobin. The study confirms that SpHb is accurate, reliable, and a clinically-acceptable alternative for monitoring hemoglobin, and is the first SpHb study presented in pediatric patients.
Dr. Fay Jou and colleagues at the Cincinnati Children's Hospital Medical Center in Ohio compared SpHb and point-of-care (POC) hemoglobin measurements (Abbott iStat™) to a standard laboratory hematology analyzer (Abbott Cell Dyn hematology analyzer) in 15 pediatric patients undergoing surgery. Compared to standard laboratory hemoglobin measurements, SpHb and iStat had a similar bias (-0.3 and 0.2 g/dL respectively) and standard deviation (1.1 and 0.5 g/dL, respectively). Researchers concluded that "SpHb offers clinically-acceptable absolute accuracy and very good trend accuracy" and that all significant directional changes in hemoglobin "were indicated by changes in SpHb." Additionally, researchers commented that "SpHb provided earlier indications of directional hemoglobin changes than intermittent tHb values."1
According to Dean Kurth, Anesthesiologist in Chief at Cincinnati Children's Hospital Medical Center, "These study results indicate that Masimo SpHb has the potential to replace the need for invasive blood draws in infants and children undergoing surgery."
SpHb is available as part of Masimo Rainbow SET Pulse CO-Oximetry—the first-and-only technology platform to noninvasively measure blood constituents and fluid responsiveness that previously required invasive procedures, including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa™), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI). Masimo SpHb and PVI have been shown in multiple clinical studies to provide accurate, reliable, real-time measurements that help clinicians to proactively monitor and manage hemoglobin and fluid volume levels more appropriately and optimally.
1 F. Jou, C. Kurth, E. Beckman, G.K. Istaphanous. Department of Anesthesiology, Cincinnati Children's Hospital Medical Center. "Absolute and Trend Accuracy of Continuous and Noninvasive Hemoglobin in Pediatric Surgery Patients." Presentation S-401, Monday, March 22, 2010, 11 a.m. to 12:30 p.m.; International Anesthesia Research Society (IARS), Honolulu, Hawaii.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Rainbow SET Pulse CO-Oximetry technology will provide sufficient sensitivity and specificity to accurately measure SpHb, SpOC, PVI, SpCO, SpMet, RRa, SpO2, PI, and PR in real-time for all patients, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Dartmouth-Hitchcock Medical Center Receives ECRI Institute Award for Innovative Patient Monitoring Project Using Masimo SET Pulse Oximetry and Masimo Patient SafetyNet™
Irvine, California – March 19, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, is pleased to report that Dartmouth-Hitchcock Medical Center (Lebanon, New Hampshire) has received the 4th Annual ECRI Institute Health Devices Achievement Award yesterday for an innovative patient monitoring project that uses its Masimo SET pulse oximetry and Masimo Patient SafetyNet remote monitoring and clinician notification system. The award-winning project enabled clinicians to significantly improve post-surgical patient outcomes and reduce hospital costs by $817,000 in its first year.1 In their announcement of the award, the ECRI Institute honored Dartmouth-Hitchcock for "the most exceptional example of an initiative undertaken by an ECRI Institute member to improve patient safety, reduce costs, or otherwise facilitate better strategic management of health technology." ECRI Institute's Vice President of Health Technology Evaluation and Safety, Jim Keller, presented the award this morning to Jeanne Avery, Senior Clinical Quality Specialist, and Ken Lee, Clinical Manager, Biomedical Engineering, at Dartmouth-Hitchcock.
"We are very proud of this award and the accomplishments and environment of care that it represents," stated George Blike, M.D., Quality and Patient Safety Officer, Dartmouth-Hitchcock Medical Center. "Creating excellence in patient care today requires both a high-tech and high-touch approach. We believe that together, Masimo's innovative technologies and our implementation truly made a difference, which is incredibly rewarding for everyone involved."
Joe Kiani, Chairman and CEO of Masimo, stated, "We are delighted to see the ECRI Institute recognize Dartmouth-Hitchcock Medical Center for their excellence in implementing and managing health technology to drive improved patient outcomes, safety, and cost savings. Researchers and clinicians at Dartmouth-Hitchcock should all be congratulated for their visionary work, its potential to advance the standard and quality of care, and its impact on healthcare for all patients. They prove that great technology in the hands of caring clinicians can make a huge difference in not only the lives of patients, but in lowering the cost of healthcare."
ECRI Institute, an independent nonprofit that researches the best approaches to improving patient care, selected Dartmouth-Hitchcock for excellence in health technology management based on their initiative to decrease failure to rescue events—instances of severe patient harm (such as death or disability) that occur because a serious deterioration in the patient's condition is not detected in time. Dartmouth-Hitchcock's winning healthcare initiative, "A Multidisciplinary Approach to Improving Patient Safety in the Adult Medical/Surgical Population through Earlier Detection of Patient Deterioration Using Surveillance Monitoring," leverages a new application of pulse oximetry—continuously monitoring post-surgical patients on the general floor from admission to discharge—using a combination of advanced medical technologies—Measure-Through Motion and Low Perfusion pulse oximetry with Masimo SET and automatic clinician notification with Masimo Patient SafetyNet. The implementation resulted in 65% fewer rescue events and 48% fewer ICU transfers, which freed up 135 ICU-days annually for more critically-ill patients.2
Dartmouth-Hitchcock's award-winning project is featured in the March 2010 issue of Health Devices, available at:https://www.ecri.org/Products/Pages/Health_Devices_Journals.aspx.
1 J.A. Morgan, A.H. Taenzer, S.P. McGrath, G.T. Blike. Dartmouth-Hitchcock Medical Center. "Cost-effectiveness of Patient Surveillance Systems." Presentation S-249, Monday, March 22, 2010, 9-10:30 a.m.; International Anesthesia Research Society (IARS), Honolulu, Hawaii. For further reading, visit: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?mID=2481&sKey=00f42012-e254-4e88-9828-f8cc6e370103&cKey=9c549b96-143a-4aae-a1e3-370031a85e57&mKey=%7bBA0F4D4C-6673-49B4-9438-7D745CEA2C01%7d.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading, visit: http://journals.lww.com/anesthesiology/Abstract/publishahead/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.99692.aspx.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
About ECRI Institute
ECRI Institute, a nonprofit organization, dedicates itself to bringing the discipline of applied scientific research to healthcare to discover which medical procedures, devices, drugs, and processes are best to enable improved patient care. As pioneers in this science for 40 years, ECRI Institute marries experience and independence with the objectivity of evidence-based research. ECRI Institute is designated a Collaborating Center of the World Health Organization and an Evidence-based Practice by the U.S. Agency for Healthcare Research and Quality. ECRI Institute PSO is listed as a federally certified Patient Safety Organization by the U.S. Department of Health and Human Resources. For more information, visit https://www.ecri.org.
About Dartmouth Hitchcock
Dartmouth-Hitchcock Medical Center (DHMC) is New Hampshire's only academic medical center. Internationally renowned and nationally ranked, DHMC serves a population of 1.5 million in Vermont and New Hampshire. DHMC integrates high-quality patient care, advanced medical education, and translational research to provide a full spectrum of health care. Dartmouth-Hitchcock Medical Center includes Norris Cotton Cancer Center, a National Cancer Institute designated Comprehensive Care Center, and is home to the Children's Hospital at Dartmouth. The center also includes Mary Hitchcock Memorial Hospital (396-bed teaching hospital), the Dartmouth-Hitchcock Clinic, Veterans Affairs Regional Medical and Office Center, and Dartmouth Medical School. For more information on Dartmouth-Hitchcock Medical Center, visit www.dhmc.org.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical and financial results, risks related to our assumptions that automatic clinician notifications provided by Masimo Patient SafetyNet will enable timely rescue, risks related to our belief that continuous monitoring with Masimo SET pulse oximetry will detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, and risks related to our assumptions regarding the system's ability to deliver clinical improvement over alternative patient monitoring/assessment methods, decrease rescue events and ICU transfers, and improve both patient safety and outcomes, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Carol Kocher
ECRI Institute
(610) 825-6000 ext. 5377
ckocher@ecri.org
Annette Moore / Victoria McCandless
Dartmouth-Hitchcock Medical Center
(603) 653-1967 / (603) 653-1941
Annette.E.Moore@hitchcock.org / Victoria.L.McCandless@hitchcock.org
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Cost-Effectiveness Study Shows Hospitals Could Save More Than $1Million Annually with Masimo Patient SafetyNet™
Data Presented at the International Anesthesia Research Society Calculates Savings at Over $250 per Patient
Irvine, California – March 17, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that a new cost-effectiveness study shows that implementation of the Masimo Patient SafetyNet remote monitoring and clinician notification system with Masimo SET pulse oximetry saved one hospital $817,000 in its first year. The study, being presented on Monday, March 22, 2010, at the International Anesthesia Research Society (IARS) Annual Meeting in Honolulu, Hawaii, also projects that the hospital's future annual savings will be $1,295,000—providing a compelling financial rationale to expand monitoring in post-surgical patients on the general floor.1
In the study titled: "Cost-effectiveness of Patient Surveillance Systems," researchers at Dartmouth-Hitchcock Medical Center in Lebanon, NH, analyzed the cost savings associated with clinical outcome improvements shown in their clinical study published in the February 2010 issue of Anesthesiology—the first published report to demonstrate that continuous Measure-Through Motion and Low Perfusion pulse oximetry monitoring of post-surgical patients on the general floor with Masimo SET and automatic clinician notification with Masimo Patient SafetyNet leads to a "significant drop" in key clinical outcome measures, including fewer rescue events, fewer ICU transfers, and reduced annualized ICU days.2 After comparing cost data gathered before and after installation on Dartmouth's 36-bed post-surgical general care unit, researchers showed that implementation of Masimo Patient SafetyNet enabled clinicians to reduce hospital costs by $255 per patient the first year and a projected $404 per patient in subsequent years. Researchers concluded that these findings could "aid hospital administrators and physician leadership in their decision to deploy patient surveillance systems."
In the past, four primary factors prevented many hospitals from utilizing continuous pulse oximetry monitoring in post-surgical patients on the general floor: 1) An un-manageable number of false alarms; 2) costly and overly complex patient monitoring solutions; 3) the lack of clinical outcomes' data demonstrating improved patient safety; and 4) the lack of clear financial justification. Today, the false alarm problem has been solved with gold-standard Masimo SET pulse oximetry—proven to reduce false alarms by 95%. The costly and overly complex problem has been solved with Masimo Patient SafetyNet—offering easy-to-use, but powerful remote monitoring that leverages the hospital's existing wireless network, requires only minimal equipment at the bedside, and does not require central station monitoring. And now, both unprecedented clinical outcomes and cost savings have been attained by utilizing Masimo SET and Masimo Patient SafetyNet together on the general floor.
According to Steve Moreau, President and CEO, San Antonio Community Hospital in Upland, California, "This study quantifies what we have believed all along—that rapid response and the prevention of costly transfers to ICU saves both lives and dollars. It's clear that the annual cost savings enabled by the Masimo Patient SafetyNet System could be significantly impactful on an individual hospital's bottom-line, but may also have bigger implications for healthcare economics overall."
Under the financial model established by researchers, if all 5,815 registered hospitals in the U.S. were to implement Patient SafetyNet and realize the cost savings attained in the study, it would save between $4.7 and $7.5 billion in healthcare expenses each year—representing a compelling financial justification for the U.S. healthcare system.
Michael O'Reilly, MD, EVP of Medical Affairs at Masimo, stated, "Researchers at the Dartmouth-Hitchcock Medical Center have continued their groundbreaking clinical research into the patient and hospital impact of implementing Masimo technologies for post-surgical patients on the general floor with this cost-effectiveness study. Although many new technologies improve patient care, they typically do so at an increased cost. However, as this new study shows, Masimo SET and Masimo Patient SafetyNet have transcended the cost barrier to become part of a very select group of technologies that improve clinical outcomes and provide net cost savings. In today's challenging healthcare climate, these benefits cannot be underestimated."
1 J.A. Morgan, A.H. Taenzer, S.P. McGrath, G.T. Blike. Dartmouth-Hitchcock Medical Center. "Cost-effectiveness of Patient Surveillance Systems." Presentation S-249, Monday, March 22, 2010, 9-10:30 a.m.; International Anesthesia Research Society (IARS), Honolulu, Hawaii. For further reading, visit: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?mID=2481&sKey=00f42012-e254-4e88-9828-f8cc6e370103&cKey=9c549b96-143a-4aae-a1e3-370031a85e57&mKey=%7bBA0F4D4C-6673-49B4-9438-7D745CEA2C01%7d.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading, visit: http://journals.lww.com/anesthesiology/Abstract/publishahead/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.99692.aspx.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of study results, risks related to our assumptions that Masimo Patient SafetyNet will provide an effective early warning system of a patient's deteriorating physiological condition to enable timely rescue, risks related to our belief that Masimo Rainbow SET Pulse CO-Oximetry measurements and Masimo Desat Index 3D Alarms will provide sufficient sensitivity and specificity to detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, and risks related to our assumptions regarding the systems' ability to deliver clinical improvement over alternative patient monitoring and assessment methods to increase patient safety and allow for further adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Owensboro Medical Health System Converts System-wide to Masimo Pulse Oximetry to Improve Patient Monitoring Capabilities
Cutting-Edge Medical Technology Advancements Help a Regional Healthcare Provider Serving Western Kentucky and Southern Indiana to Improve Patient Monitoring, Safety, and Quality
Irvine, California – March 11, 2010 – Owensboro Medical Health System, a HealthGrades Distinguished Hospital for Clinical Excellence Award™ recipient, and Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today jointly announced the completion of Owensboro Medical Health System's system-wide conversion to Masimo pulse oximetry technology. The conversion ensures that Owensboro patients will be monitored using the most technologically and clinically-advanced pulse oximetry and Pulse CO-Oximetry technologies available—providing real-time results for vital measurements that help clinicians to more rapidly assess, diagnose, and treat patients.
"The decision to convert our entire network to Masimo pulse oximetry technology came down to three goals: improving the accuracy, quality, and safety of our patient monitoring capabilities," said Mike Sisley, Manager of Respiratory Care at Owensboro Medical Health System. "It became very clear during the evaluation process that only Masimo allowed us to accomplish all three, and yet, at a cost savings. And with critical measurements like noninvasive hemoglobin, we saw firsthand how Masimo could help us to advance patient care."
The system-wide conversion standardizes all of Owensboro's sites of care, including over 400 hospital beds, to Masimo technology, which involved upgrading the hospital's multiparameter patient monitors, pulse oximeters, and sensors. Masimo offers the only upgradable pulse oximetry technology platform—Masimo Rainbow SET Pulse CO-Oximetry—that allows hospitals to add breakthrough noninvasive blood constituent measurement capabilities that previously required invasive procedures. The ability to continuously and noninvasively measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa™), in addition to Masimo SET 'gold standard' Measure-Through Motion and Low Perfusion oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR) measurements enables monitoring of multiple physiological parameters simultaneously—to help clinicians detect life-threatening conditions and help guide treatment options.
According to Masimo Executive Vice President of Medical Affairs, Michael O'Reilly, MD, "Owensboro Medical Health System's decision to convert to Masimo technology will help clinicians to more accurately and closely monitor patients in greater clinical detail. The noninvasive and continuous measurement of key physiological parameters facilitates identification of abnormalities that may provide an earlier warning of declining health status."
About Owensboro Medical Health System
Owensboro Medical Health System serves an 11-county region in Western Kentucky and Southern Indiana. The hospital received the HealthGrades Distinguished Hospital for Clinical Excellence Award in 2009, placing it among the top five percent of hospitals in the nation for quality. OMHS is a full-service hospital, employing a workforce of nearly 3,200. OMHS also operates the HealthPark, a medical-based fitness center and a number of ancillary services, clinics and diagnostic centers. Regional clinics include MultiCare locations in Madisonville and Beaver Dam (KY) and Tell City (IN). OMHS also provides care through the Clinic at Walmart, in Owensboro, Henderson (KY) and Newburgh (IN).
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Patient SafetyNet will provide an effective early warning system of a patient's deteriorating physiological condition in real-time for all patients to enable timely rescue and increase patient safety, risks related to our belief that Masimo SET and Masimo Rainbow SET measurements and alarms will provide sufficient sensitivity and specificity to detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Gordon Wilkerson
Owensboro Medical Health System
(270) 685-7194
gwilkerson@omhs.org
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at Roth Capital 22nd Annual OC Growth Stock Conference
IRVINE, Calif., March 8, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that its management is scheduled to present at the Roth Capital 22nd Annual OC Growth Stock Conference at The Ritz-Carlton in Dana Point, Calif. on Monday, March 15, 2010, at 9:00 a.m. PT. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Masimo Corporation
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at Cowen and Company 30th Annual Health Care Conference
IRVINE, Calif., March 2, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that its management is scheduled to present at the Cowen and Company 30th Annual Health Care Conference at The Boston Marriott Copley Place on Wednesday, March 10, 2010, at 11:00 a.m. ET. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET® provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI® and acoustic respiration rate (RRa™), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Masimo Corporation
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Lutheran Medical Center in New York Invests in State-of-the-Art Masimo Patient SafetyNet™ System to Help Keep Patients Safer
System Noninvasively Monitors Physiology of up to 80 Patients on Four Floors—Helping Clinicians Improve Safety, Speed Recoveries, Increase Efficiencies, and Reduce the Cost of Care
Irvine, California – February 25, 2010 – Lutheran Medical Center in Brooklyn, New York and Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced the installation of the Masimo Patient SafetyNet system, an advanced noninvasive remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events—one of today's most common medical errors.1
Masimo Patient SafetyNet allows clinicians at Lutheran Medical Center to noninvasively, continuously, and remotely monitor multiple physiological parameters—including arterial oxygen saturation, respiration rate, hemoglobin levels, and pulse rate—and have changes that signal early patient distress or deterioration automatically sent via pager to a qualified caregiver. Earlier detection may facilitate earlier interventions, which has been shown to help improve outcomes. In a recently-published landmark study at Dartmouth-Hitchcock Medical Center, the Masimo Patient SafetyNet system was shown to help decrease distress codes and activations by 65%, and intensive care unit (ICU) transfers by 48%—resulting in an annualized savings of 135 ICU days.2
"Patient SafetyNet provides the peace of mind that comes from knowing that it is always watching over our patients—continuously monitoring their physiology when we can't be at the bedside to do it," said Helen Buckel, RN, Nurse Manager, Lutheran Medical Center. "Even when we leave the room, we know that little finger sensor the patient wears is doing an enormous job. The moment the patient encounters trouble or a life-threatening event occurs, the system immediately alerts us, so precious seconds aren't wasted and lifesaving help is just a beat away."
When a patient is in distress, specific, detailed physiological data is sent directly to assigned clinicians, enabling them to intervene before the situation becomes critical and requires more acute levels of care. This is particularly important for post-surgical patients who are at increased risk of injury or death resulting from the respiratory depression effects of medications used for sedation and pain management.
Masimo Executive Vice President of Medical Affairs, Michael O'Reilly, MD, stated, "Each year healthy patients admitted for routine procedures become failure to rescue statistics because an adverse event was not recognized in time to save them. Masimo Patient SafetyNet significantly expands the patient monitoring capabilities available to Lutheran clinicians, helping to improve patient outcomes and decrease costs—two things every hospital wants and needs."
1 "The Sixth Annual HealthGrades Patient Safety in American Hospitals Study" April 2009.
http://www.healthgrades.com/media/DMS/pdf/PatientSafetyInAmericanHospitalsStudy2009.pdf
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. http://journals.lww.com/anesthesiology/Abstract/publishahead/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.99692.aspx
About Lutheran Medical Center
A Level I Trauma Center and Stroke Center, Lutheran Medical Center (LMC) has cared for Brooklyn communities since 1883. As a full service 476-bed teaching hospital, LMC is the hub of Lutheran HealthCare, a network of primary, acute and long-term care centers in southwest Brooklyn. The LMC Surgical Weight Loss Institute is the only program in Brooklyn to be designated as a Center of Excellence by the American Society for Bariatric Surgery while also holding a Level 1 Accreditation from the American College of Surgeons. Learn more about LMC and its commitment to patient care excellence, community service, health education and research online at www.LutheranMedicalCenter.com.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Patient SafetyNet will provide an effective early warning system of a patient's deteriorating physiological condition in real-time for all patients to enable timely rescue and increase patient safety, risks related to our belief that Masimo SET and Masimo Rainbow SET measurements and alarms will provide sufficient sensitivity and specificity to detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Nicole Hyland
Lutheran Medical Center
(718) 630-7414
nhyland@lmcmc.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Board of Directors Declares Special Cash Dividend
Dividend Set at $2.00 Per Share
Irvine, California – February 22, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that its Board of Directors has declared a special $2.00 per share cash dividend, payable on March 31, 2010 to stockholders of record as of the close of business on March 11, 2010. The total dividend payout is expected to be about $116 million, based on the current shares outstanding and will be the second instance of a special dividend paid by Masimo in the past four years.
Masimo Founder, CEO and Chairman of the Board, Joe Kiani, stated, "I am very proud that, based on the company's strengthening financial position, the Board has taken this action for the immediate benefit of stockholders. While dividends are not routine for our company, our healthy balance sheet and strong cash flow, along with our expectations for continued growth, have contributed to the Board's confidence in declaring this special dividend."
Mr. Kiani continued, "As part of our ongoing technological evaluation process, including a review of potential business acquisitions, we have determined that our current focus should remain on pursuing the enormous opportunities that SET, Rainbow and Patient SafetyNet have created for our company and stockholders. While we will continue to consider new opportunities, we believe that, for now, there is no business we would rather focus on than our own superior healthcare solutions that yield benefits for patients, practitioners and payers as evidenced by the rising adoption of these innovative technologies in multiple settings throughout the world."
Over the past year, Masimo's Board evaluated a variety of options to return capital to shareholders, including acquisition opportunities and a stock buy-back program before concluding that a special dividend was the best and most direct way to reward shareholders for their continued investment and confidence in Masimo. The company previously paid special dividends to shareholders totaling $4.09 a share related to the 2006 intellectual property patent suit settlement agreement with a competitor. While there can be no guarantees of future dividends, the Masimo Board remains committed to enhancing shareholder value based on its consideration of various factors, including the company's operating results, financial condition and anticipated capital requirements.
This special dividend represents only a portion of the company's cash reserves, which the Board believes is sufficient to cover operational needs, and fund continued investments in research and development and strategic initiatives. The company remains committed to its previously announced programs to intensify marketing and clinical research efforts this year using funds awarded from recent legal decisions.
Since the company's founding, Masimo has remained true to its mission of 'improving patient outcomes and reducing the cost of care by taking noninvasive monitoring to new sites and applications.' The company is realizing this mission as its breakthrough SET Pulse Oximetry is now enabling continuous monitoring of patients in new sites such as the general floor and converting invasive measurements into noninvasive measurements such as total hemoglobin and carboxyhemoglobin with its Rainbow platform. Caregivers rely upon these breakthroughs today to help them provide the most advanced standard of care possible. Masimo's vision remains to bring SET Pulse Oximetry monitoring to the entire hospital, including the general floor, and to make Rainbow noninvasive hemoglobin monitoring as ubiquitous as pulse oximetry. This has also enabled Masimo to deliver double-digit product revenue growth every year since it first shipped product in 1996, with a five year CAGR of 34%.
Mr. Kiani concluded, "Because of the benefits our products provide to caregivers and patients, we have established a track record of revenue and earnings growth that has put Masimo in the strongest financial position of its history. The Board has decided to declare this special dividend because we remain confident in our future and our ability to grow organically, generate cash and provide increasing value to stockholders."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Rainbow SET Pulse CO-Oximetry technology will provide sufficient sensitivity and specificity to measure SpHb, SpOC, PVI, SpO2, SpCO, SpMet, PI, and PR in real-time for all patients, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Investor Contacts:
Sheree Aronson
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Published Study Demonstrates Patients with Low Hemoglobin Levels Can be Managed Without Blood Transfusions
Findings Suggest the Current Practice of Single Hemoglobin Transfusion Triggers Leads to Unnecessary Transfusions and a Better Physiological Basis for Transfusion Decisions is Warranted
Irvine, California – February 18, 2010 – A comparison study of nearly 1,900 patients published in Perfusion, a peer-reviewed academic journal, questions the long-standing practice of basing blood transfusion decisions on a single low hematocrit measurement result. In the study, titled Is it the Patient or the Physician who Cannot Tolerate Anemia?, researchers showed that a single measure of hematocrit is not a reliable indicator of transfusion need and that lower-than-normal hemoglobin levels have no adverse impact on patients. The study showed that a blood transfusion is largely administered because the physician deems it necessary, not because of quantified changes in the patient's physiology.
The study prospectively analyzed the outcomes of 1,854 patients with high (>21%) and low (≤21%) hematocrit levels who underwent coronary artery bypass graft surgery without receiving red blood cells at any time during their hospital stay. In comparing outcomes between the two groups, researchers found that the rates were similar in both groups regarding time on ventilator, duration of intensive care unit stay, intensive care unit re-admission, hospital re-admission, reoperation for bleeding or tamponade, low cardiac output, postoperative atrial fibrillation, stroke, creatinine level at hospital discharge, new onset renal failure, mediastinitis, pulmonary complication, and mortality rates. The study results showed that hematocrit levels that are considered "low" (between 17-21%) "are well tolerated and have no adverse impact on outcome," leading researchers to conclude "it is the physician, not the patient, who cannot tolerate low hematocrit levels."1
Despite mounting clinical evidence linking adverse patient outcomes to blood transfusions during cardiac surgery, including increased operative mortality and decreased long-term survival,3,4 almost half of patients undergoing coronary artery bypass in the U.S. still receive at least one unit of packed red blood cells.5,6 In the Perfusion study researchers contend that the decision to unnecessarily transfuse blood in cardiac surgery patients is often based solely on a single low hematocrit level of below 20-22%—a clinical practice more than a half century old.
According to the American Society of Anesthesiologists (ASA) practice guidelines: "red blood cell transfusions should not be dictated by a single hemoglobin 'transfusion trigger' but instead should be based on the patient's risk of developing complications of inadequate oxygenation."2
In fact, Dr. Aryeh Shander, President-Elect of the Society for the Advancement of Blood Management (SABM) and the Executive Medical Director for The Institute for Patient Blood Management & Bloodless Medicine and Surgery at Englewood Hospital and Medical Center in Englewood, New Jersey, says that "transfusion guidelines such as the ASA's are becoming increasingly important for the clinician. The transfusion decision is quite complex because of mounting data surrounding risks and negative outcomes coupled with the unproven benefit of red cell transfusions. Deciding to transfuse based on a single static measurement more often results in patients receiving unnecessary transfusions with increased risks, costs and the depletion of an already scarce blood supply. New medical technologies and devices that continuously monitor hemoglobin, oxygen, and perfusion will become essential for transfusions."
One of the early pioneers of blood conservation and bloodless surgery techniques, Dr. Thomas Crimi, a founding member of SABM, Director of the Blood Conservation Program at Brookdale University Medical Center, and long-time proponent of single transfusion avoidance believes that "the lack of enabling medical technology to accurately and continuously measure key physiological parameters such as hemoglobin, fluid responsiveness, tissue oxygenation, and perfusion simultaneously has historically led physicians to make transfusion decisions based on single hemoglobin value reflecting measures obtained at a single point in time. However, today with Masimo Pulse CO-Oximeters, I get the real-time hemoglobin, oxygenation, tissue perfusion, and fluid responsiveness measurements and trending data that I need," he confirmed.
Masimo Rainbow SET Pulse CO-Oximetry—a breakthrough noninvasive blood constituent monitoring platform capable of measuring multiple blood constituents that previously required invasive procedures, including: total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI). Masimo SpHb, PVI, and SpO2 have been shown in multiple clinical studies to provide accurate, reliable, real-time measurements that help clinicians to proactively monitor and manage hemoglobin, fluid, and oxygen saturation levels more appropriately and conservatively.
1 Senay S, Toraman F, Karabulut H, Alhan C. "Is it the Patient or the Physician who Cannot Tolerate Anemia? A Prospective Analysis in 1854 Non-transfused Coronary Artery Surgery Patients." Perfusion, November 2009; Vol. 24(6):373-80. Available online: http://prf.sagepub.com/cgi/content/abstract/24/6/373
2 American Society of Anesthesiologists, "Practice guidelines for blood component therapy: a report by the American Society of Anesthesiologists Task Force on Blood Component Therapy." Anesthesiology 1996; 84: 732–747.
3 Surgenor SD, DeFoe GR, Fillinger MP, et al. "Intraoperative Red Blood Cell Transfusion During Coronary Artery Bypass Graft Surgery Increases the Risk of Postoperative Low-output Heart Failure." Circulation 2006; 114: I43-I48.
4 Murphy GJ, Reeves BC, Rogers CA, Sizvi SIA, Culliford L, Angelini GD. "Increased Mortality, Postoperative Morbidity, and Cost after Red Blood Cell Transfusion in Patients Having Cardiac Surgery." Circulation 2007; 116: 2544-2552.
5 Rawn JD. "Blood Transfusion in Cardiac Surgery: A Silent Epidemic Revisited." Circulation 2007; 116: 2523-2524.
6 Koch CG, Li L, Duncan AI, et al. "Morbidity and Mortality Risk Associated with Red Blood Cell and Blood-Component Transfusion in Isolated Coronary Artery Bypass Grafting." Crit Care Med 2006; 34: 1608-1616.
About SABM
The Society for the Advancement of Blood Management (SABM) is an educational organization comprised of a network of practitioners from a wide variety of medical and scientific disciplines who are dedicated to improving patient outcomes and the advancement of optimal patient blood management in clinical practice through education, cooperation and research. The society works to facilitate cooperation among existing and future patient blood management/blood conservation, bloodless medicine and surgery programs, as well as to enhance the clinical and scientific aspects of transfusion practice. Patient blood management seeks to optimize and conserve the patient's own blood, reducing or avoiding the need for a blood transfusion. Stored donor blood should only be used as therapy, with patient consent, when there are no alternatives, and when the expected benefits exceed the negative consequences. Additional information is available at: www.sabm.org.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Rainbow SET Pulse CO-Oximetry technology will provide sufficient sensitivity and specificity to measure SpHb, SpOC, PVI, SpO2, SpCO, SpMet, PI, and PR in real-time for all patients, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports Fourth Quarter and Full Year 2009 Financial Results
Fourth Quarter 2009 Highlights:
- Product revenues of $80.5 million, up 12% compared to the fourth quarter last year
- Rainbow revenues of $5.8 million, up 23% compared to the fourth quarter last year
- Shipped 30,400 Masimo SET and Masimo Rainbow SET pulse oximeter units
- Diluted EPS of $0.23
Full Year 2009 Highlights:
- Product revenues of $300.1 million, up 16% compared to 2008
- Rainbow revenues of $19.5 million, up 46% compared to 2008
- Shipped over 111,000 Masimo SET and Masimo Rainbow SET pulse oximeter units in 2009
- Diluted EPS of $0.88
Irvine, California, February 16, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, today announced its financial results for the fourth quarter and year ended January 2, 2010.
Masimo's fourth quarter product revenues rose 12% to $80.5 million, compared to $71.6 million for the fourth quarter of 2008. Revenues from Masimo Rainbow SET products rose 23% to $5.8 million in the fourth quarter. Including royalty revenues, Masimo's total revenues for the fourth quarter were $92.6 million, up 11% from $83.1 million for the fourth quarter of 2008.
Net income for the fourth quarter was $14.1 million, or $0.23 per diluted share, compared to a net loss of approximately $530,000, or $0.01 per share in the same period last year, which included a $14.9 million, or $0.25 per share, tax charge related primarily to the prepayment of licensing rights to utilize pre-existing intangibles as part of the implementation of a new international business organization and structure.
For 2009, Masimo product revenues rose 16% to $300.1 million, compared to $259.6 million in 2008. Revenues from Masimo Rainbow SET products rose 46% to $19.5 million in 2009. Including royalty revenues, 2009 total revenues were $349.1 million, up 14% from $307.1 million in 2008. Total 2009 direct and distribution revenues were $241.7 million, up 20% from $201.1 million in 2008 while OEM revenues were unchanged at $58.4 million.
Masimo reported 2009 net income of $53.2 million, or $0.88 per diluted share, compared to $31.9 million, or $0.53 per diluted share in 2008, which included the $0.25 per share tax charge related to the prepayment of licensing rights to utilize pre-existing intangibles as part of the implementation of its new international business organization and structure.
During the fourth quarter, the company shipped 30,400 Masimo SET pulse oximetry and Masimo Rainbow SET Pulse CO-Oximetry units, excluding handheld units, compared to 31,700 in the same period last year. Based on a 10-year average useful life assumption, the company estimates its worldwide installed base as of January 2, 2010 to be approximately 724,000 units, compared to approximately 625,000 for the same period last year.
Joe E. Kiani, Chairman and Chief Executive Officer of Masimo, said, "We focus on inventing and delivering breakthrough noninvasive vital signs monitors that help clinicians make better and faster decisions in order to improve the quality and efficiency of patient care. Our fourth quarter and full year 2009 financial results show the fundamental strength of our innovation engine and business model. We attribute our sustained growth to the power of our core Masimo SET pulse oximetry platform, combined with our Masimo Rainbow SET Pulse CO-Oximetry offering, which is extending use of noninvasive measurements to new applications such as carboxyhemoglobin, methemoglobin, hemoglobin and beyond."
As of January 2, 2010, cash, cash equivalents and short-term investments totaled $189.0 million, up 29% from $146.9 million at January 3, 2009.
Financial Guidance
Masimo expects fiscal 2010 total revenues to be between $390 million and $405 million, including product revenues between $345 million and $360 million, and royalty revenues between $44 million and $46 million. The company expects fiscal 2010 earnings per share to be between $1.12 and $1.18, including approximately $0.15 from a one-time gain related to resolution of an anticompetitive legal matter with Covidien in the first quarter of 2010, offset by expected incremental one-time spending associated with this recovery. The company's guidance also includes an increase in 2010 non-cash stock-based compensation expense to $14.7 million from $10.7 million in 2009. The components of Masimo's guidance set forth above are estimates only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation code for both dial-in numbers is 52590350. A live webcast of the conference call will be available online from the "Investor Relations" page of the company's corporate website at www.masimo.com. After the live webcast, the call will remain available on Masimo's website through March 16, 2010. In addition, a telephonic replay of the call will be available through March 2, 2010. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 52590350.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations, prospects and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and expectations for total revenues, including royalty revenues, product revenues and Rainbow revenues, non-cash stock based compensation charges, and GAAP earnings per share for the full fiscal year 2010. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo Rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any new products and technologies of ours; obtaining regulatory approval of our current and future products and technologies, including the recently announced total hemoglobin measurement; the risk that the implementation of our international realignment, will not produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Masimo Corporation
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57, Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
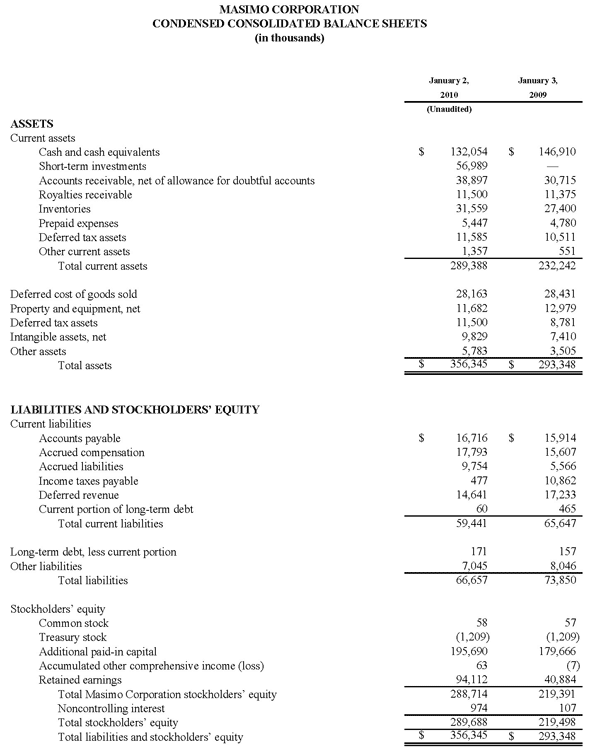
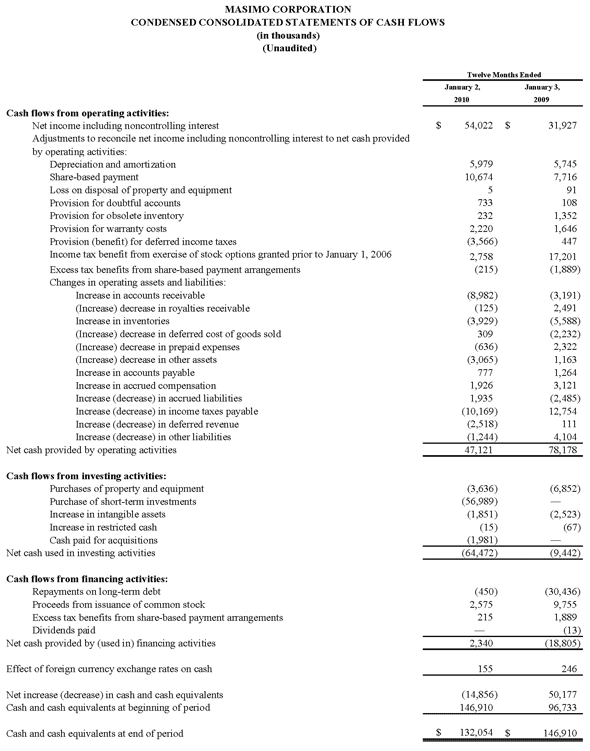


Masimo to Report Fourth Quarter and Full Year 2009 Financial Results After Market Close on February 16, 2010
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., February 2, 2010 -- Masimo Corporation (Nasdaq: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced it will release fourth quarter and full year 2009 financial results for the period ended January 2, 2010 after the market closes on February 16, 2010.
A conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe E. Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation code for both dial-in numbers is 52590350. After the live webcast, the call will be available on Masimo's website through March 16, 2010. In addition, a telephonic replay of the call will be available through March 2, 2010. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 52590350.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa™), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Masimo Corporation
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


St. Luke's Episcopal Hospital Becomes First in Texas to Monitor Post-surgical Patients with Masimo Patient SafetyNet™
Clinically-Proven Technology Helps St. Luke's Clinicians Improve Patient Care and Reduce Costs
Houston, Texas – January 26, 2010 – St. Luke's Episcopal Hospital (SLEH) and Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, jointly announced today that the Houston-based hospital is the first in Texas to use the Masimo Patient SafetyNet™ system to continuously monitor the physiological status of post-surgical patients—ensuring early detection of patient deterioration and enabling potentially life-saving interventions.
With the Patient SafetyNet system, St. Luke's clinicians receive a pager notification when a patient's condition is worsening, allowing them to intervene before the condition becomes critical and requires more acute levels of care. This is particularly important for post-surgical patients who are at increased risk of serious injury or death resulting from the respiratory depression effects of patient-controlled analgesia (PCA) and opioids used for sedation and pain management.
According to John Sabo, administrative director of Respiratory Therapy at St. Luke's Episcopal Hospital, Masimo Patient SafetyNet is helping St. Luke's to realize its vision of improving both patient safety and hospital efficiencies through early detection and intervention of acute respiratory changes in its post-surgical patients. "We have already witnessed the benefits of Patient SafetyNet in our orthopedic and pulmonary units. Patient SafetyNet will assist in the improvement of utilization of ICU resources, patient outcomes, and a reduction in overall costs."
The Patient SafetyNet system keeps post-surgical patients safer by continuously, noninvasively, and remotely monitoring multiple physiological parameters, including arterial oxygen saturation and pulse rate, and automatically alerting clinicians to changes that signal patient distress or deterioration. In a recently published landmark study by Dartmouth Hitchcock Medical Center, the Patient SafetyNet system was shown to help reduce rescue events and activations 65% and ICU transfers 48%.1
"Once my alarm limits are set, I know I can rely on Patient SafetyNet to watch over my patients, continuously assessing their health status 24/7," stated Samantha Biba, BSN, RN-BC, Acute Pulmonary Unit, Quality Supervisor. "At the first sign of trouble, the system alerts me via pager so I can attend to the patient and assess his or her condition. Not only has the system been instrumental in helping us to identify patient deterioration much earlier to prevent more serious adverse events, but its effect on patient care and safety has been invaluable."
Combining performance of Masimo SET® pulse oximetry with respiration rate monitoring at the point-of-care and wireless clinician notification via pager, Patient SafetyNet provides an unmatched level of safety for up to 80 patients on four floors. The system uses open IEEE industry standards for connectivity, which allows for more efficient sharing of data across a hospital's IT platforms, along with the option of full integration into a hospital's existing IT infrastructure—providing a lower overall cost of ownership and improved financial benefits.
To further advance patient safety initiatives, St. Luke's will also implement Masimo Rainbow SET Pulse CO-Oximetry technology—making noninvasive and continuous hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI® an integral component of their routine patient monitoring protocols in their emergency, surgery, and recovery care areas.
1 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2.
Available online at: http://journals.lww.com/anesthesiology/Abstract/publishahead/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.99692.aspx
About St. Luke's Episcopal Health System
St. Luke's Episcopal Health System (www.stlukestexas.com) includes St. Luke's Episcopal Hospital in the Texas Medical Center, founded in 1954 by the Episcopal Diocese of Texas; St. Luke's The Woodlands Hospital; St. Luke's Sugar Land Hospital; St. Luke's Lakeside Hospital; St. Luke's Episcopal Health Charities, a charity devoted to assessing and enhancing community health, especially among the underserved. St. Luke's Episcopal Hospital is home to the Texas Heart® Institute, which was founded in 1962 by Denton A. Cooley, MD, and is consistently ranked among the top 10 cardiology and heart surgery centers in the country by U.S.News & World Report. Affiliated with several nursing schools and three medical schools, St. Luke's Episcopal Hospital was the first hospital in Texas named a Magnet hospital for nursing excellence, and has been honored four times with the Distinguished Hospital Award for Clinical Excellence™ by HealthGrades, a leading independent company that measures healthcare quality in hospitals. The Health System has been recognized by FORTUNE as among the "100 Best Companies to Work For" and by the Houston Business Journal as a top employer in Houston. St. Luke's Episcopal Health System also was honored as one of Modern Healthcare magazine's "100 Best Places to Work."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Patient SafetyNet will provide an effective early warning system of a patient's deteriorating physiological condition to enable timely rescue and increase patient safety, risks related to our belief that Masimo SET and Masimo Rainbow SET measurements and alarms will provide sufficient sensitivity and specificity to detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Jessica Michan
St. Luke's Episcopal Health System
(832) 355-3791
jmichan@sleh.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Landmark Study Shows Masimo SET® Pulse Oximetry and Patient SafetyNet™ Can Help Hospitals Dramatically Decrease Rescue Events and ICU Transfers, and ICU Days
Irvine, California – January 22, 2010 – Dartmouth-Hitchcock Medical Center and Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, jointly announced the peer-reviewed publication of an in-depth, 21-month clinical study on the impact of the Masimo Patient SafetyNet remote monitoring and clinician notification system. The study, featured in the February 2010 issue of Anesthesiology, is the first published report to demonstrate that continuous pulse oximetry monitoring and clinician notification in post-surgical patients on the general floor leads to a "significant drop" in key clinical outcome measures, including 65% fewer rescue events, 48% fewer ICU transfers, and reduced annualized ICU time by 135 days.1
In the study, Dr. Andreas Taenzer and a team of clinicians at Dartmouth-Hitchcock Medical Center used Masimo Patient SafetyNet—which combines the gold-standard performance of Masimo SET pulse oximetry at the point of care with remote monitoring and wireless clinician notification via pager—in a 36-bed post-surgical orthopedic unit. When comparing data collected for 11 months before and 10 months after implementation Patient SafetyNet in the 36-bed unit – as well as two other post-operative units with only standard monitoring equipment and protocols in place - researchers found that Patient SafetyNet-monitored patients experienced approximately 65% fewer rescue events (1.2 vs. 3.4 per 1,000 patient discharges) and 48% fewer ICU transfers (2.9 vs. 5.6 per 1,000 patient days)—freeing up 135 ICU days per year, while the two comparison units had no change.
"Masimo Patient SafetyNet represents a new approach to detect unrecognized post-operative deterioration—a significant precursor in morbidity and mortality for in-hospital patients," stated the lead researcher and author of the study, Andreas H. Taenzer, MD, FAAP, Assistant Professor of Anesthesiology and Pediatrics at the Dartmouth Medical School, Dartmouth-Hitchcock Medical Center, in Lebanon, New Hampshire. "Our study results strongly demonstrate that continuous patient surveillance with Masimo Patient SafetyNet can greatly improve outcomes."
In an accompanying editorial about the impact of the study, John P. Abenstein, MSEE, MD, at the Department of Medicine, Mayo Clinic, in Rochester, Minnesota, wrote that the "implications of this study are broad" and its results could "have important implications for hospital wards throughout the country."2 According to Dr. Abenstein, "The literature and each of our own clinical experiences have examples of physicians on rounds, or nurses coming to check patients who have been dead for hours." He continued, "We believe that Taenzer et al. have shown us a glimpse of the future" and "Not only will such systems allow us to improve the quality of care of our patients, but will also be a key to lowering costs."
Masimo Founder and CEO, Joe Kiani, stated, "This is one of the most important studies ever published on pulse oximetry because it shows that with Masimo SET Pulse Oximetry technology, improving patient safety and reducing the cost of care can go together. There have been other studies that have shown the positive clinical and cost outcome in neonates and infants by using Masimo SET pulse oximetry, but this is the first time with adults. Over 20 years ago, we set out to solve the motion artifact and low perfusion problems of pulse oximetry, which were the bane of pulse oximetry and were thought to be unsolvable at the time. We thought by overcoming the motion artifact problem, we could improve patient outcome and reduce cost of care by taking noninvasive monitoring to new sites and applications. In fact, this became our mission statement. The clinicians at Dartmouth-Hitchcock, with their culture of patient safety, have shown a breakthrough in patient care is not only possible, but can be attained cost effectively. With this groundbreaking study, our vision is that in the near future, hospitals will utilize this important Patient SafetyNet technology to care for all of their patients, the same way airbags have become ubiquitous in cars today."
1 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2.
Available online at: http://journals.lww.com/anesthesiology/Abstract/publishahead/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.99692.aspx
2 Abenstein, John P.; Narr, Bradly J. "An Ounce of Prevention May Equate to a Pound of Cure: Can Early Detection and Intervention Prevent Adverse Events?" Anesthesiology, February 2010, Vol. 112, Issue 2. Available online at: http://journals.lww.com/anesthesiology/Citation/publishahead/An_Ounce_of_Prevention_May_Equate_to_a_Pound_of.99693.aspx
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of study results, risks related to our assumptions that Masimo Patient SafetyNet will provide an effective early warning system of a patient's deteriorating physiological condition to enable timely rescue, risks related to our belief that Masimo Rainbow SET Pulse CO-Oximetry measurements and Masimo Desat Index 3D Alarms will provide sufficient sensitivity and specificity to detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, and risks related to our assumptions regarding the systems' ability to deliver clinical improvement over alternative patient monitoring and assessment methods to increase patient safety and allow for further adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


BMEYE Receives CE Mark to Launch First Noninvasive Cardiovascular Monitor with Masimo Rainbow SET® Pulse CO-Oximetry™ Technology in Europe
Incorporating Masimo's Breakthrough Noninvasive Hemoglobin and Oxygen Saturation Measurements, ccNexfin Debuts to European Market
Amsterdam, The Netherlands and Irvine, California – January 21, 2010 – BMEYE B.V., the innovators of combined noninvasive, beat-to-beat blood pressure and cardiac output monitoring, and Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low-Perfusion pulse oximetry, jointly announce today CE Mark certification and the European launch of the BMEYE ccNexfin—the first noninvasive cardiovascular monitor with Masimo Rainbow SET Pulse CO-Oximetry technology. The combination of two innovative noninvasive technologies—BMEYE for cardiovascular monitoring and Masimo Rainbow SET for hemoglobin and oxygen saturation monitoring—provides real-time, beat-to-beat measurements of cardiac, circulatory, and pulmonary parameters, which may enable clinicians to detect impending cardiovascular crisis before organ injury ensues.
The ccNexfin with Masimo Rainbow SET offers some of the most advanced cardiovascular monitoring capabilities available today. It utilizes a single sensor finger cuff (BMEYE) and finger sensor (Masimo Rainbow SET) to capture and continuously measure beat-to-beat blood pressure (sys/dia), mean arterial pressure (MAP), cardiac output (CO), stroke volume (SV), systemic vascular resistance (SVR), derivative of pressure (dP/dt), noninvasive hemoglobin (SpHb), oxygen saturation (SpO2), and perfusion index (PI). This detailed data allows clinicians to predict and proactively address the early signs of hemodynamic instability during critical situations rather than reacting to late indicators and their effects.
"ccNexfin is a unique noninvasive, beat-to-beat, and user friendly cardiovascular monitor that delivers a patient's full hemodynamic profile based on real-time data," stated Rob de Ree, CEO of BMEYE. "Masimo Rainbow SET Pulse CO-Oximetry plays an integral role in the delivery of this rich hemodynamic data stream by providing critical information about oxygen delivery at the tissue level, which is an essential component of accurate hemodynamic assessment."
Assessment of hemodynamic status is a cornerstone of critical care medicine, routinely used to evaluate, diagnose, and direct therapy to manage the care of at-risk and critically-ill patients. Hemodynamic monitoring—the direct measurement of blood pressure inside the veins, heart and arteries in relation to cardiac output and systemic vascular resistance—allows clinicians to assess the cardiovascular system and identify early circulation changes that reflect small changes in the way the heart is working to deliver blood and oxygen to tissues and organs.
Hemodynamic monitoring has traditionally relied upon invasive, costly, intermittent, or unreliable methods to assist clinicians in maintaining adequate oxygen delivery and tissue perfusion to prevent hypoxia and its irreversible damage. The integration of ccNexfin and Masimo Rainbow SET technologies into the emergency department, intensive care units, and anesthesiology care areas has the potential to facilitate earlier diagnosis and the delivery of protocol- and goal-directed therapies that improve patient safety and clinical outcomes.
Masimo Founder and CEO, Joe Kiani, stated, "The risks and costs associated with invasive hemodynamic monitoring techniques leaves many high-risk patients underserved at a time when assessing hemodynamic stability is most needed. The synergistic combination of BMEYE and Rainbow SET means that continuous, noninvasive hemodynamic monitoring can benefit more patients at all points along the care path."
About BMEYE
BMEYE, a Dutch company based in Amsterdam, The Netherlands, develops innovative medical devices with broad application in clinical settings that require cardiovascular monitoring. Specifically, BMEYE produces patient monitors that noninvasively measure continuous blood pressure and cardiac output. BMEYE's mission: BMEYE is the premier provider and will set the standard of noninvasive, beat-to-beat, user-friendly cardiovascular monitoring systems, to improve patient care and reduce healthcare costs.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the timing and market availability of ccNexfin with Masimo Rainbow SET, risks related to our assumptions that the integration of Masimo Rainbow SET with ccNexfin provides advanced noninvasive and continuous hemodynamic monitoring capabilities that have the potential to improve patient safety and outcomes by assisting in the delivery of protocolized, goal-directed therapies, risks related to our belief that the combination of Masimo Rainbow SET and ccNexfin will enable clinicians to detect impending cardiovascular crisis before organ injury ensues, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


U.S. Department of Homeland Security Issues Federal Warning About Duty-related Carbon Monoxide (CO) Dangers for Firefighters
FED Warns: Even Mild CO Poisoning Can Rob the Brain and Heart of Vital Oxygen— Causing Life-threatening Complications
Irvine, California – January 19, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that the U.S. Department of Homeland Security recently issued a Federal warning about the increased risk of carbon monoxide exposure during the winter season—urging heightened awareness and precaution for firefighters, who are at increased risk of duty-related CO danger. The life-threatening consequences of CO poisoning make immediate detection, helped by the noninvasive Masimo Rad-57 Pulse CO-Oximeter, a lifesaving necessity for fire and emergency operations.
The Department of Homeland Security's Emergency Management and Response Information Sharing and Analysis Center (EMR-ISAC) INFOGRAM 1-10, warns that "below freezing temperatures now occurring at many locations throughout the United States potentially increase the risk for carbon monoxide exposure" as people try to use alternative sources for heat. According to the Federal warning, firefighters responding to fires and emergency calls "should understand that CO poisoning is a danger at every fire, regardless of its cause" and "its symptoms – headache, dizziness, fatigue – are often absent or non-specific, making on-scene awareness and detection difficult." Detection is important because it "puts firefighters at significant risk because even mild CO poisoning can deny the brain of oxygen. It can also rob the heart of oxygen, causing immediate life-threatening complications."
Carbon monoxide is a colorless, odorless, and tasteless toxic gas that is extremely difficult to detect—making it the leading cause of poisoning in industrialized countries. Prior to Masimo Rainbow SET Pulse CO-Oximetry, an invasive blood draw followed by laboratory blood gas analysis was the only reliable method for diagnosing CO poisoning. Without immediate access to measure CO in the blood, emergency first responders were at a critical disadvantage. Today, the portable, handheld Masimo Rad-57 Pulse CO-Oximeter provides an accurate and noninvasive way to detect elevated CO levels in the bloodstream in just seconds—helping emergency first responders to quickly and easily diagnose CO poisoning on-the-scene and initiate prompt, lifesaving treatment.
The full warning can be found at: http://www.usfa.dhs.gov/downloads/pdf/infograms/1_10.pdf
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced Masimo Rainbow SET® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's Rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of case results, risks related to our belief that Masimo Pulse CO-Oximetry/SpCO/Rad-57 provides unique clinical advantages as an immediate, accurate, reliable early detector of elevated CO blood levels, and risks related to our assumptions that Masimo SpCO represents a more rapid, reliable and cost-effective clinical alternative for CO screening, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Published Study Finds Masimo PVI® Predicts Fluid Responsiveness as Accurately as Invasive Stroke Volume Variation in Mechanically-Ventilated Patients
Masimo PVI Accuracy Shown to Be Superior to Invasive Central Venous Pressure
Irvine, California – January 14, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that a new study published in the January 2010 issue of the European Journal of Anaesthesiology found that Masimo PVI predicts fluid responsiveness as accurately as invasive stroke volume variation (SVV) and more accurately than invasive central venous pressure (CVP) in mechanically-ventilated patients undergoing major surgery.1
Although fluid administration is critical to optimizing patient status and enabling end organ preservation, unnecessary fluid administration is associated with increased morbidity and mortality. Traditional invasive measurements such as CVP are not reliable to predict whether a patient will benefit from fluid administration, and newer more accurate methods to predict fluid responsiveness, such as SVV, are invasive and costly. Because SVV has been shown in multiple studies to be an accurate method to predict fluid responsiveness and optimize fluid management in surgical and intensive care patients, the similar accuracy shown with noninvasive PVI indicates that PVI-guided fluid optimization may also help guide fluid management to minimize patient risk and improve outcomes.
In the study, Dr. Zimmermann and colleagues at University Hospital Regensburg in Germany simultaneously measured PVI (obtained noninvasively using the Masimo Radical-7 Pulse CO-Oximeter), arterial pressure-based SVV (obtained invasively via radial arterial line using the Edwards Lifesciences FloTrac/Vigileo system), and CVP (obtained invasively via central venous catheter), in 20 patients immediately after induction of anesthesia and again after volume administration (6% hydroxyl-ethyl starch). Patients were classified as responders if stroke volume index (SVI), the amount of blood the heart pumps in each heartbeat, increased by 15% or more, and as non-responders if SVI did not increase 15%.
Results showed that a PVI value greater than 9.5% predicted fluid responsiveness with 93% sensitivity and 100% specificity, an SVV value greater than 11% predicted fluid responsiveness with 100% sensitivity and 80% specificity, while a CVP value greater than 10.5 mm Hg predicted fluid responsiveness with 66% sensitivity and 40% specificity.
The researchers concluded that both PVI and SVV "can serve as valid indicators of fluid responsiveness in mechanically-ventilated patients undergoing major surgery," but only PVI "seems to provide a simple alternative for accurate, noninvasive, and continuous preload monitoring."
Masimo Executive Vice President of Medical Affairs Dr. Michael O'Reilly, stated, "Unnecessary fluid administration can increase the risk of serious injury or death, making fluid responsiveness monitoring a growing requirement to advance patient safety. The researchers at the University Hospital Regensburg have further demonstrated that accurate fluid responsiveness does not require invasive procedures, demonstrating that Masimo PVI is a safe, reliable, and noninvasive alternative to guide fluid management to minimize patient risk and improve outcomes."
1 Markus Zimmerman, Thomas Feibicke, Cornelius Keyl, Christopher Prasser, Stefan Moritz, Bernhard M. Graf, and Christoph Wiesenack. "Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically-ventilated Patients Undergoing Major Surgery." European Journal of Anaesthesiology 2010; Vol 27, No 00.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa™), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo PVI provides unique clinical advantages as an automatic noninvasive measurement of fluid responsiveness, risks related to our assumptions that Masimo PVI is as accurately as invasive stroke volume variation (SVV) and more accurate than invasive central venous pressure (CVP) in mechanically-ventilated patients, and risks related to our assumptions that Masimo PVI represents a more rapid, reliable and cost-effective clinical alternative for assessing fluid responsiveness and help optimize fluid management in surgical and intensive care patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Announces Investment in SedLine Brain Function Monitoring Business
Irvine, California – January 12, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced a strategic investment totaling $3.5 million in SedLine, Inc., a newly-formed private company that was formerly operated by Hospira, Inc. Under terms of the investment agreement, Masimo may increase its investment subject to certain financial and development milestones. SedLine's mission is to expand the scope and applications for neuromonitoring. Masimo's investment will allow SedLine to continue to support its existing customers, market to new customers, and expand its research and development efforts.
Masimo Chairman and CEO, Joe Kiani, stated, "Multiple leading hospitals in the United States have already chosen SedLine because they believe, as Masimo does, that SedLine's current technology is better than competitive brain function monitoring technologies. Masimo has always been a strong advocate for innovation, patient care, and providing choice in the marketplace, so we are happy to become an investor in SedLine's continued commercial availability and support this new entity in their goal to enhance and expand the applications for brain function monitoring."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET Pulse CO-Oximetry continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. In 2009, Masimo introduced Rainbow SET Acoustic Monitoring, offering continuous and noninvasive respiration rate (RRa™) from an innovative acoustic sensor applied to the patient's neck. Rainbow Acoustic Monitoring is accurate, easy-to-use, and enhances patient compliance – which may help clinicians detect respiratory compromise and patient distress earlier and offer a breakthrough in patient safety. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
About SedLine
SedLine, Inc. is a research-focused company with the mission to expand the scope and applications for neuromonitoring. Based on over 10 years of technical and clinical development, the SedLine core product is a pioneering four-channel brain-function monitor that measures the effects of anesthesia and sedation by simultaneously analyzing, quantifying and displaying both sides of the brain's electrical activity. The SedLine monitor is currently in use at some of the nation's leading healthcare institutions. Privately held and based in Irvine, California, SedLine has an active R&D effort underway to develop advanced brain function monitoring technologies for next generation monitors to further improve the care of patients under anesthesia or sedation.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to the factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Receives Payment Following Court of Appeals Affirmance of Antitrust Liability Verdict Against Tyco HealthCare
Payment is on ruling finding that Tyco, now Covidien, unlawfully maintained monopoly power and utilized unlawful restraints of trade and exclusive dealing arrangements in the pulse oximetry market
Irvine, California – January 11, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that it retained $30,064,684 from a payment from Covidien, following the Ninth Circuit Court of Appeals' October 2009 affirmance of a Federal District Court decision that Tyco Healthcare, now Covidien, violated the antitrust laws through anticompetitive business practices related to the sale of its pulse oximetry products. The decision found that Covidien had unlawfully maintained monopoly power in violation of Section 2 of the Sherman Act, and that Covidien's sole-source agreements and market-share based compliance pricing contracts constituted unlawful restraints of trade in violation of Section 1 of the Sherman Act and unlawful exclusive dealing in violation of Section 3 of the Clayton Act. The Ninth Circuit also stated that above-cost bundling discounts when combined with sole-source or market-share–based compliance contracts can be anticompetitive when such practices involve a significant portion of the market. The suit was originally filed by Masimo in 2002. The judgment against Covidien for the antitrust violations was for $43.5 Million; however, the total payment, after reimbursement for legal fees, costs, and interest was $58,982,215. The portion of the total payment from Covidien that was not retained by Masimo was paid to the law firm that handled the trial for Masimo.
Some confusion seems to have occurred in the last few days as a result of another ruling by the Ninth Circuit Court of Appeals in January 2010 in the Allied Orthopedic Appliances' case against Covidien. The Allied Orthopedic case has no impact on the finding of antitrust liability in the Masimo case. As noted by the District Court Judge in the Allied Orthopedic decision when referencing the Masimo antitrust case against Covidien, "the instant case [referring to Allied Orthopedic] is 'entirely different' and focuses on overcharges paid by customers on pulse oximetry consumables as a result of Tyco's [now Covidien] foreclosure of generic sensor manufacturers."
Joe E. Kiani, Founder and CEO of Masimo, stated: "We are happy to have received the payment, but we are hopeful that the results that we have fought will be served, which is to help improve patient care while also reducing cost by improving caregivers' access to cost-effective, innovative products. This ruling is the result of one of many efforts Masimo has pursued for many years to open markets so that medical products are judged on their merits rather than artificial restraints on hospital purchasing. Opening competition in the pulse oximetry market has caused pulse oximetry pricing to decrease by over 30%. But, also, many people's lives were either saved or improved as a direct result of their access to Masimo pulse oximeters. "
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET Pulse CO-Oximetry continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. In 2009, Masimo introduced Rainbow SET Acoustic Monitoring, offering continuous and noninvasive respiration rate (RRa™) from an innovative acoustic sensor applied to the patient's neck. Rainbow Acoustic Monitoring is accurate, easy-to-use, and enhances patient compliance – which may help clinicians detect respiratory compromise and patient distress earlier and offer a breakthrough in patient safety. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to the factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Noninvasive Carbon Monoxide (CO) Screening with Masimo Pulse CO-Oximetry™ Helps Detect and Treat Victims While Identifying Source of CO Poisoning
Newly Published Case Study Shows Masimo SpCO® Helped Avoid Disaster—Saving Transport and Hospital Costs After Incident at Manufacturing Plant
Irvine, California – January 5, 2010 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Pesrfusion pulse oximetry, today announced that a new case study, published in the January-March 2010 issue of Prehospital Emergency Care, showed that noninvasive screening with Masimo carboxyhemoglobin (SpCO) provided emergency first responders with immediate and accurate detection of CO-poisoned employees and enabled the identification of the source of the poisoning at a manufacturing plant. The early detection and subsequent identification of the CO source helped avert a potential disaster that may have injured or cost the lives of numerous employees and overwhelmed both the local emergency medical services (EMS) system and area hospitals.1
Carbon monoxide is a colorless, odorless, and tasteless toxic gas that is extremely difficult to detect—making it the leading cause of poisoning in industrialized countries. Prior to Masimo Rainbow SET Pulse CO-Oximetry, an invasive blood draw followed by laboratory blood gas analysis was the only reliable method for diagnosing CO poisoning. Without immediate access to measure CO in the blood, emergency first responders were at a critical disadvantage. Today, the portable, handheld Masimo Rad-57 Pulse CO-Oximeter provides an accurate and noninvasive way to detect elevated CO levels in the bloodstream in just seconds—allowing emergency first responders to quickly and easily diagnose CO poisoning on-the-scene and initiate prompt, lifesaving treatment.
The published case report describes how a suburban fire department and EMS unit in Rock Springs, Georgia responded to a call for a sick employee at a local manufacturing plant. The first responders combined the use of both atmospheric CO testing and Pulse CO-Oximetry to successfully diagnose the cause of the employee's illness and identify the source of the poisoning. Upon arrival, firefighters initially used an atmospheric gas monitor to measure the amount of CO gas circulating in the air and found that the air inside the plant contained elevated, unsafe levels of CO (between 26-70 PPM). However, firefighters searching the building were unable to identify its source. All employees working inside the plant were evacuated and screened for CO poisoning by measuring their SpCO levels using the Masimo Rad-57. Although the source of the poisoning was still unknown, firefighters promptly treated employees with elevated SpCO levels—ranging from 5-18%—on the scene with high concentration oxygen.
While the most obvious source appeared to be a recently installed industrial furnace in the foundry area, no detectable atmospheric CO levels were present anywhere in the area. Although atmospheric CO levels were highest in the assembly area, there were no CO sources in that part of the building. In a second attempt to locate the source of the poisoning, firefighters marked the locations of the employees with the highest SpCO levels on a map of the plant and found that they were all working immediately under air conditioning vents in the assembly area. After tracing the air conditioning vents, firefighters determined that exhaust from the furnace in the foundry were being drawn into a nearby intake vent for the air conditioning system.
According to the lead author of the case study, Bryan E. Bledsoe, DO, FACEP, "The measurement of SpCO levels via Pulse CO-Oximetry provided the missing link to a potentially disastrous puzzle. When initial atmospheric CO monitoring missed the mark—revealing high levels in an area where no CO source existed and the absence of CO in the area where the likely source was found—Masimo SpCO allowed emergency responders to instantly detect biological CO levels, initiate treatment of those in need, and promptly map the exposure point. This potentially saved countless lives while avoiding needless transport of large numbers of employees to area hospitals, preventing significant emergency department and EMS costs—and possibly leaving others in the community without emergency services."
Each year, the lifesaving accounts from fire departments and EMS professionals around the world showcase the importance of the Masimo Rainbow SET Pulse CO-Oximeter to their public safety efforts—helping to save lives, limit long-term brain and heart damage, and create efficiencies and cost savings in the care and delivery of emergency medical services. Most recently, thanks to the Masimo Rainbow SET Pulse CO-Oximeter and swift emergency intervention, more than 50 motel occupants and staff in North Carolina, over 40 families in Beijing, and numerous families in Alabama, Illinois, Colorado, and Washington were saved from becoming carbon monoxide-poisoning statistics.
Masimo Executive Vice President of Medical Affairs Dr. Michael O'Reilly, stated, "Whether suspected or unsuspected, CO poisoning presents a unique challenge to firefighters and EMS personnel who need to know immediately if elevated CO levels are in the bloodstream. However, because CO levels in the air of a particular area don't always correlate to the amount of poisoning in the blood and vice versa, Masimo SpCO is a vital compliment to atmospheric CO monitoring to help first responders save lives, avert mass casualty incidents, and alleviate cost and care burdens on the local emergency system."
1 Bryan E. Bledsoe, Kevin Nowicki, James H. Creel Jr., Dale Carrison, Harry W. Severance. "Use of Pulse Co-Oximetry as a Screening and Monitoring Tool in Mass Carbon Monoxide Poisoning." Prehospital Emergency Care January-March 2010, Vol. 14, No. 1 : Pages 131-133
Available online at: http://tinyurl.com/spco-averts-mass-casualty
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa™), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of case report results, risks related to our belief that Masimo SpCO provides unique clinical advantages as an immediate, accurate, reliable early detector of elevated CO blood levels, and risks related to our assumptions that Masimo SpCO represents a more rapid, reliable and cost-effective clinical alternative for CO screening, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
