News & Media-2009
Home / 2009
2009
NEWS & MEDIA:

St. Vincent Hospital Renews Multi-Year, System-wide Contract for Masimo SET® Pulse Oximetry Technology
Indianapolis-Based Health Provider Adds Six More Years to its Masimo SET Pulse Oximetry Standardization Contract
Irvine, California – December 30, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that St. Vincent Indianapolis Hospital has renewed its campus-wide contract for Masimo SET pulse oximetry technology. A Masimo customer since 2000, St. Vincent cites advanced monitoring technology, an upgradable platform, and innovative new capabilities as key reasons for the six-year system-wide renewal of their technology standardization contract, which will continue to support the oximetry needs of the quaternary facility.
"All pulse oximetry technologies are not created equal," stated Kathy Gatons, BA, RRT, Director of Clinical Support at St. Vincent Hospital. "Masimo offers the best, most advanced oximetry technologies available. We know the measurements can be trusted and the alarms are valid when a Masimo pulse oximeter is on the patient. That's why we chose to standardize on Masimo SET 10 years ago and why we continue to leverage Masimo as our pulse oximetry platform for the future. Patient safety is a huge initiative that we support by placing Masimo technology at every patient bedside."
Continuing their standardization on Masimo SET pulse oximetry technology allows St. Vincent Hospital to leverage the unique breakthrough noninvasive blood constituent monitoring capabilities of Masimo Rainbow SET Pulse CO-Oximetry. Masimo Rainbow SET is the first-and-only technology platform capable of continuously and noninvasively measuring multiple blood constituents and parameters that previously required invasive procedures, including: total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI® for fluid responsiveness, and acoustic respiration rate (RRa™), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI). The ability to immediately detect and treat potentially life-threatening conditions without having to draw blood and wait for the results facilitates earlier and better clinical decisions that may help St. Vincent clinicians to save lives and improve patient outcomes.
According to Rex McKinney, MBA, RRT, CHP, FACHE, Executive Director, Clinical Support Services at St. Vincent Hospital, "Masimo has excelled at providing leading technology that we need to support our patient safety initiatives. St. Vincent places the highest priority on providing healthcare that is safe. The Masimo technology platform provides our clinicians with the tools to monitor patients effectively and provide the safest clinical setting possible."
Masimo Founder and CEO, Joe E. Kiani, stated, "As an early adopter of Masimo SET technology, St. Vincent was one of the first hospitals to see the clinical value and patient impact of Masimo technology. Today, their unwavering commitment to delivering the best possible patient outcomes and remaining on the front line of medical advances is as strong as ever. And, we are happy that they have chosen to bring the latest noninvasive technologies to the patients they serve with Masimo Rainbow SET—helping provide faster, more accurate diagnosis, treatment, and recovery to those in need."
St. Vincent Hospitals and Health Services
Driven by the faith of four Daughters of Charity who arrived in Indianapolis in 1881 with $34.77 in their pockets, the St. Vincent Hospital mission is to treat the poor and sick by following our Core Values of Service of the Poor, Reverence, Integrity, Wisdom, Creativity and Dedication. Our healthcare ministry has grown to include seven Centers of Excellence: Women's, Children's, Orthopedics, Cardiovascular, Neuroscience, Cancer Care and Bariatrics. The ageless mission of St. Vincent remains unchanged: to minister to the minds, bodies and spirits of those in need.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SET and Masimo Rainbow SET will provide sufficient sensitivity and specificity to detect physiological abnormalities in real-time—enabling clinicians to detect and identify potentially life-threatening conditions earlier and make better clinical and treatment decisions that may help save lives and improve outcomes for their patients, risks related to our assumption that the system-wide renewal of St. Vincent Health Care will serve to substantially increase revenues, and risks related to our assumptions regarding the future of the St. Vincent Health Care system-wide pulse oximetry renewal contract, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Johnny Smith
St. Vincent Health
(317) 583-3961
jasmith1@stvincent.org
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Rainbow SET® SpHb™ Measurement Receives New CPT Code and Medicare Pricing
Irvine, California – December 29, 2009 – Masimo, the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that the American Medical Association has granted a new Current Procedural Terminology (CPT) code, 88738, for Masimo's noninvasive hemoglobin (SpHb) measurement. Medicare has priced the CPT code at $7.19 on the 2010 Medicare Clinical Laboratory Fee Schedule, effective January 1, 2010. The new CPT code and pricing will allow healthcare providers to bill and receive payment when testing eligible patients.
1 2010 Medical Clinical Laboratory Fee Schedule. Available at http://www.cms.hhs.gov
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa™) in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the establishment of CPT codes for Masimo SpHb will increase the application and broaden the use of Masimo Rainbow SET Pulse CO-Oximetry technology or serve to provide substantially increased revenues for the company, and risks related to our assumptions that SpHb will deliver a sufficient level of clinical improvement over alternative measurement capabilities to allow for rapid adoption of the technology, risks related to our assumptions regarding the repeatability of clinical results, risks related to our assumptions that Masimo SpHb is an accurate, reliable and acceptable clinical alternative to invasive blood tests, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Expands its Irvine, California Corporate Headquarters to Accommodate Growth Projections
December 24th Open House Showcases the Addition of a New Building to the Corporate Campus and New Health Care & Wellness Plan For Masimo Employees
Irvine, California – December 24, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced the expansion of its Irvine, California headquarters with the addition of an adjacent 32,000 sq. ft. building—the company's third building at its corporate campus. Expanding its corporate headquarters is a natural progression for Masimo, which continues to post double-digit growth increases. The company hosted an open house on Thursday, December 24th, from 11 a.m. to 2 p.m., to celebrate the new building and the launch of the Masimo Health Care and Wellness Program.
The expansion is further proof that, despite a slumping U.S. economy, Masimo continues to buck national economic trends, reporting product revenue growth of 17% during the first nine months of 2009 compared to the first nine months of 2008. The new building is expected to house more than 200 employees, many of which the company anticipates adding to its engineering, sales, and administrative functions in 2010 to accommodate the growing demand for its breakthrough medical technologies, including the expansion of its Masimo Rainbow SET Pulse CO-Oximetry and new Acoustic Monitoring business.
Today, Masimo SET, is at work in the world's most demanding hospitals, with more than half of the top U.S. hospitals—including four of the top five, as listed on the US News & World Report Honor Roll—adopting Masimo SET as their primary pulse oximetry platform. This year alone, more than 150 hospitals throughout the world have converted to Masimo's advanced pulse oximetry technology.
In addition to celebrating the expansion of its corporate campus, Masimo employees also celebrated the unveiling of a new 2010 Masimo Wellness Plan—an employee-controlled healthcare solution designed to promote wellness and incentivize employees to lead healthy lifestyles. The plan provides the umbrella protection and safety net of a traditional PPO health insurance plan with 100% coverage for preventative care—making it easier and more cost-effective for employees to get routine physicals, annual exams, wellness check-ups and immunizations to stay healthy.
"Over the last year, U.S. healthcare reform has taken center stage. And, as a medical technology innovator on the forefront of improving care and safety for patients, we at Masimo understand just how crucial it is for our nation to get healthcare right," explained Masimo Founder and CEO Joe Kiani. "We know that millions of Americans are depending on it, and billions of people around the world are watching us! That's why we have been an active member in both local/community and national discussions concerning healthcare reform in America. We felt a duty to represent the 'patient care' side of the healthcare story and doing so allowed us to learn best practices from the nation's most forward-thinking organizations."
Kiani explained that what struck Masimo about what these forward-thinking organizations were doing was that they were building insurance plans that put more control and responsibility in the hands of their employees.
"They allowed their employees to navigate and chart their own course to better health—leading the charge for healthcare reform within their own organizations while lowering its costs," Kiani added. "This inspired us to 'do what is best for patient care,' starting with our own employees!"
Masimo believes that these efforts will pay off by helping to improve the health of its employees and by reducing the immediate and long-term costs for the company and its employees. For example, the total cost of our U.S. group insurance benefits in 2009 was in excess of $6 million, and was expected to rise further in 2010. According to Kiani, the decision to implement a comprehensive Wellness Plan will allow Masimo to keep its costs flat for 2010 and will likely reduce costs for future years.
The company is so convinced that healthier people mean a healthier company that it is planning on putting money on it with a new Wellness Rewards Program slated to begin in 2011. Under the program, employees will earn cash rewards for reaching optimal health goals, like maintaining a healthy body mass index (BMI), keeping blood pressure and cholesterol levels in normal ranges, even smoking cessation. The company believes that incentivizing employees to "shape up and save" will not only lead to healthier bodies and lifestyles, but healthier minds and savings too.
"We're excited to be in a position to spread this sort of holiday cheer this year. For many businesses, the prospect of growth within the current economy is a far stretch," Kiani concluded. "Yet, thankfully for us, our unique technology and product offerings allow us to benefit from market demand and expansion opportunities that make growth a reality that we are actively planning for despite the many obstacles. I am grateful that we are able to provide the lifesaving and life-changing medical technologies that clinicians and patients all over the world depend on, and the job growth and employment opportunities that our economy needs."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa™), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: the certainty of future growth and market opportunities, expectations for future business expansion, and job growth and employment opportunities facilitated by business growth and/or the addition of the building located at 60 Parker, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at the 28th Annual J.P. Morgan Healthcare Conference
IRVINE, Calif., December 23, 2009 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that its management is scheduled to present at the 28th Annual J.P. Morgan Healthcare Conference at the Westin St. Francis Hotel in San Francisco on Monday, January 11, 2010, at 2:00 p.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET® provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI® and acoustic respiration rate (RRa), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Masimo Corporation
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Happy Holidays from Masimo!
Irvine, California—December 22, 2009—This has been another exciting year for us here at Masimo, as we commemorated the 20th anniversary of our founding, and continued to see clinicians use our technologies to improve patient safety and enhance the quality of patient care in around the world. It was a year in which we celebrated the commercial launch of the world's first noninvasive and continuous hemoglobin monitoring technology and the debut of the world's first acoustic respiration rate monitoring technology. It is at this very special time of year that we look to share our blessings by making a special donation on your behalf to a charitable organization that needs our help the most.
As we look to the future, we are encouraged by the successes of the past and driven by the desire to continue working with the clinical community to facilitate even greater advancements in patient care in the years to come. It is with great appreciation for our customers, partners, shareholders and employees that we close out this year by making a donation of $10 to the charity of your choice. We will make this donation in the name of each person who is an official member of Livewire as of today and who responds to this Livewire with his or her charity choice from the list below:
- Amnesty International
- CARE
- Doctors Without Borders
- Huntington's DSA
- Make-a-Wish Foundation
- March of Dimes
- Opportunity International
- SOS Children's Villages
- Swan Foundation
- UNICEF
- United Way
- World Vision
Please send us an e-mail specifying your selection to: charity@masimo.com. Only requests by e-mail to this address will be processed. We also encourage you to include any comments or suggestions you might have that will help us to better fulfill our mission and adhere to our guiding principles.

Masimo Mission Statement
Improving patient outcomes and reducing cost of care by taking noninvasive monitoring to new sites and applications.
Masimo Guiding Principles
- Remain faithful to your promises and responsibilities.
- Thrive on fascination and accomplishment and not on greed and power.
- Make each day as fun as possible.
- Strive to make each year better than the year before, both personally and for the Team.
- Do what is best for patient care.
We thank each of you for your support in helping us to make life better for the clinicians and patients we diligently serve, and look forward to a new year full of great possibilities.
With sincere gratitude and warmest regards,
Masimo


Masimo Announces Transition of Chief Technical Officer to Full-Time Chief Engineer
Irvine, California – December 18, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that Ammar Al-Ali, the Company's Chief Technical Officer, will be transitioning from his current position to the full time position of Engineer Fellow, Chief Engineer of the Company. The transition will remove day-to-day management responsibility for the CTO office and allow Mr. Al-Ali to focus his efforts exclusively on the creation and development of new technologies and products for the Company.
Joe Kiani, Founder & CEO of Masimo, stated: "On behalf of the entire company, I thank Ammar for his years of service as Masimo's Chief Technical Officer and the major contributions he has made during his tenure in this role. I am delighted that he will be moving into a new full-time role with the Company that will allow him to continue to help us meet our continuing mission of improving patient outcomes and reducing the cost of care by taking noninvasive monitoring to new sites and applications. Based on Ammar's contributions to Masimo over the past 15 years, including his most recent role as CTO, we are very excited about the prospects that this new role presents for both Ammar and us."
The transition is expected to occur on or about December 31, 2009. After December 31, 2009, Joe Kiani will serve as Masimo's acting CTO while Masimo continues its search for a new Chief Technology Officer, which has already begun.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including those discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Clinical Study Finds Masimo Perfusion Index (PI) Predicts Low Systemic Blood Flow in Preterm Infants
Masimo PI May Provide an Easy, Noninvasive Method for Early Intervention and Treatment of Cardiovascular Compromise
Irvine, California – December 17, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that new research findings, published in the November 2009 issue of the Journal of Perinatology, show that Masimo perfusion index (PI) accurately predicts low superior vena cava flow (SVC) in preterm, very-low-birth-weight infants—representing a promising method for continuous and noninvasive monitoring of systemic blood flow.1
SVC flow, a measure of blood flow returning to the heart from the upper body, is useful information in the cardiovascular management of neonates because it helps determine risk for intraventricular hemorrhage, developmental impairments, morbidity, and mortality.2-4 However, detecting low SVC flow is challenging because current measurement methods are complicated, operator dependent, or can be inaccurate in certain neonatal conditions.5-6 Masimo PI provides a reliable continuous, noninvasive measure of the ratio of pulsatile blood flow to the non-pulsatile or static blood in peripheral tissue, which provides a rapid, reliable, and cost-effective method to help clinicians assess perfusion and circulatory status.
In the study, Dr. Takahashi and colleagues at the National Center for Child Health and Development in Tokyo, Japan, used the Masimo Radical pulse oximeter and Masimo LNOP NeoPt-L sensor to monitor PI in 24 preterm infants during the first 72-hours following birth. When comparing PI to echocardiography to directly measure SVC, they found that a PI of -1 min-1) with good sensitivity (88%) and specificity (86%). A total of eight out of 24 infants were confirmed to have low SVC flow, and PI positively detecting seven—leading researchers to conclude that Masimo PI is a "useful index for detecting low SVC flow in very low birth weight infants born before 32 weeks of gestation" and that "the PI should be evaluated in the cardiovascular management of preterm infants."
Masimo Executive Vice President of Medical Affairs Dr. Michael O'Reilly, stated, "The current study provides additional evidence supporting the clinical utility of Masimo PI, a reliable noninvasive measure of perfusion. Previous work demonstrated the value of Masimo PI as an early indicator of clinical deterioration in neonates and life-threatening congenital heart disease. Masimo PI has also been shown highly effective in assessing the response to painful stimuli and the proper placement of epidural and regional anesthesia. These results reinforce the value of Masimo PI to routinely assess circulatory disorders and improve detection and treatment of life-threatening conditions."
1 S Takahashi, S Kakiuchi, Y Nanba, K Tsukamoto, T Nakamura, Y Ito. "The Perfusion Index Derived from a Pulse Oximeter for Predicting Low Superior Vena Cava Flow in Very Low Birth Weight Infants." Journal of Perinatology (12 November 2009) doi:10.1038/jp.2009.159.
Available online at: http://www.nature.com/jp/journal/vaop/ncurrent/full/jp2009159a.html
2 Kluckow M, Evans N. "Low Superior Vena Cava Flow and Intraventricular Haemorrhage in Preterm Infants." Arch Dis Child Fetal Neonatal Ed 2000; 82: F188–F194.
3 Osborn DA, Evans N, Kluckow M. "Hemodynamic and Antecedent Risk Factors of Early and Late Periventricular/Intraventricular Hemorrhage in Premature Infants." Pediatrics 2003; 112: 33–39.
4 Miletin J, Dempsey EM. "Low Superior Vena Cava Flow on Day 1 and Adverse Outcome in the Very Low Birthweight Infant." Arch Dis Child Fetal Neonatal Ed 2008; 93: F368–F371.
5 Kluckow M, Evans N. "Superior Vena Cava Flow in Newborn Infants: A Novel Marker of Systemic Blood Flow." Arch Dis Child Fetal Neonatal Ed 2000; 82: F182–F187.
6 Osborn DA, Evans N, Kluckow M. "Clinical Detection of Low Upper Body Blood Flow in Very Premature Infants Using Blood Pressure, Capillary Refill Time, and Central-Peripheral Temperature Difference." Arch Dis Child Fetal Neonatal Ed 2004; 89:F168–F173.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa™), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo PI provides unique clinical advantages as an automatic noninvasive measurement of low SVC and intraventricular hemorrhage in very low birth weight, preterm infants, risks related to our assumptions that Masimo PI is a useful noninvasive indicator of perfusion, an early predictor of high illness severity in neonates, and an early detection tool for left heart obstruction, and risks related to our assumptions that Masimo PI represents a more rapid, reliable and cost-effective clinical alternative for assessing perfusion and circulatory status, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Chairman and CEO Joe Kiani Receives 2009 Frost & Sullivan CEO of the Year Award
Masimo Lauded for "An Unprecedented Ability in Understanding Customer Requirements, Developing New Technologies, Delivering Relevant Products" and "Integrity in Conducting Businessand Truthfulness in Advertising"
Irvine, California – December 15, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that Masimo Chairman and CEO Joe Kiani has been awarded the 2009 Frost & Sullivan CEO of the Year Award in the North American Patient Monitoring market. According to Frost & Sullivan, the award is presented in recognition of Mr. Kiani's "unprecedented ability in understanding customer requirements, developing new technologies, delivering relevant products, and thereby exploiting new market opportunities. In leading Masimo, he has not only made technology innovation the core of the company's philosophy but also equally emphasized protection of that innovation through patent licensing and OEM contracts with established patient monitoring manufacturing companies such as GE Medical, Philips, Zoll, Atom, Draeger, and Medtronic. While accomplishing all of these things, Masimo and Mr. Kiani have also been recognized for their integrity in conducting business and truthfulness in advertising."
In its summary outlining the reasons they chose to honor Kiani with the CEO of the Year award, Frost & Sullivan referenced his significant contributions to the industry, starting with the development of Masimo SET pulse oximetry. With the assistance of his friend and fellow engineer Mohamed Diab, Frost & Sullivan noted, "Kiani embarked on achieving the impossible goal of developing a pulse oximetry technology that provides precise, reliable measurements during low peripheral profusion and patient motion. The outcome of this endeavor was the invention of a breakthrough technology in pulse oximetry, Masimo SET, which considerably reduced false alarms and increased the instrument's capability of detecting critical conditions."
"This revolutionary discovery marked the beginning of Masimo's legacy as pulse oximetry technology innovator developing and manufacturing advanced, unique equipment each year. Under Mr. Kiani's leadership, in 2005 Masimo unveiled its second ground-breaking innovation, Masimo Rainbow SET Pulse CO-Oximetry with the advanced ability to measure several blood constituents that traditionally necessitated invasive methods," Frost & Sullivan continued. "Considerable enhancements were made in this product line with the capacity to measure carboxyhemoglobin (SpCO®) in 2005, methemoglobin (SpMet®) in 2006, and pleth variability index (PVI™) in 2007. The latest Rainbow innovation from Masimo launched in 2008 is the noninvasive total hemoglobin (SpHb™). Additionally, this novel equipment also measures pulse rate, perfusion index, and oxyhemoglobin and plays a critical role in early detection and treatment of life-threatening conditions."
Frost & Sullivan added that the key to Mr. Kiani's success as the CEO of Masimo "lies in directing his technological inventions to strategically address the unmet needs of the market" and that under his leadership, Masimo's innovations have "redefined patient monitoring systems." The award summary goes on to cite "the strategies adopted by Mr. Kiani as the CEO of Masimo have persistently created new industry standards. The novel product portfolio of Masimo has added a new dimension to noninvasive patient monitoring equipment that has considerably facilitated early detection of challenging patient conditions."
"As a dynamic entrepreneur, he has accepted technological and business challenges successfully, resolved them, and created new industry benchmarks during this process. In this context, it is relevant to mention that in 2006, Mr. Kiani's relentless efforts enabled Masimo to attain a historic victory against Nellcor division of Tyco Healthcare in one of the biggest patent infringement violation lawsuits. Despite Tyco Healthcare's stature and market dominance, Mr. Kiani won his company more than $300 million in damages setting a dramatic precedent that has changed the very face of the healthcare industry," the award summary states. "In addition, his testimony before the U.S. Senate in 2002 led to Group Purchasing Organization (GPO) reforms that help ensure clinicians have access to the best technology and that every company making effective products, competitively priced, is given a chance to compete and sell to hospitals through a GPO, not just the dominant suppliers."
In addition, Frost & Sullivan said that Mr. Kiani's "technology licensing strategy has successfully demonstrated the infallibility of patenting as a critical growth propeller for emerging medical device companies. Masimo's victory against Tyco Healthcare buttresses the importance of patenting in guarding the innovations of the emerging companies against established market participants. Furthermore, this victory also indicates the capability of emerging companies to compete with the market leaders that control the market."
Frost & Sullivan continued: "While leading innovation and industry change, Masimo and Mr. Kiani have also been guided by the highest principles and ethics, resulting in recognition by customers and partners alike for their integrity in conducting business. Masimo has also received high rankings by industry publications and professional societies for truthfulness in advertising."
"In successfully augmenting his scientific acumen, his formal university training, his business foresight, and the highest integrity, Mr. Kiani has demonstrated the characteristic virtues of a true entrepreneur. As the CEO, he has constantly assisted Masimo in taking confident, progressive strides in the highly monopolized patient monitoring equipment market," the award summary concludes. "In strategically balancing product innovation, manufacturing, licensing, and marketing, Mr. Kiani has carefully delineated the cardinal elements of an effective business model. Fostering healthy competition and transforming challenges to drivers, Mr. Kiani has emerged as the ideal choice for the 2009 Frost & Sullivan CEO of the Year Award in the North American Patient Monitoring market."
"On behalf of the entire Masimo team around the world, I am humbled to accept this award," Mr. Kiani said. "This award truly reflects the hard work and dedication of a large group of dedicated men and women at Masimo who come to work each day to solve the unsolvable, and as a result make a real difference in the lives of patients."
A complete description of this award and the multiple other awards Masimo has received is available at: http://professional.masimo.com/company/masimo/awards/
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa™), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: whether Mr. Kian's receipt of the Frost & Sullivan award as CEO or the Year will have any material affect on our business, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Receives 2009 Zenith Award at the American Association of Respiratory Care Congress
Masimo Rainbow SET® Acoustic Respiration Rate Monitoring Generates Significant Interest
Irvine, California – December 9, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that it has received the prestigious Zenith Award for equipment and service excellence from the American Association of Respiratory Care (AARC) at their 55th Annual AARC International Congress in San Antonio, Texas. Masimo Rainbow SET Acoustic Monitoring was also featured in live demonstrations at the Masimo commercial exhibit and received very favorable reviews from respiratory therapists.
For the second year in a row, Masimo has achieved AARC's top industry recognition honoring respiratory care equipment and pharmaceutical manufacturers for quality and service excellence. More than 400 companies were eligible for one of five Zenith Award honors. Award winners are chosen by the association's membership—more than 44,000 respiratory care professionals—based upon the quality of equipment and/or supplies, accessibility and helpfulness of sales personnel, as well as the company's responsiveness, service record, truth in advertising, and overall support of the respiratory care profession.
In addition, hundreds of respiratory care professionals from all over the world were among the first to see live demonstrations of Masimo Rainbow SET Acoustic Monitoring—providing noninvasive and continuous respiration rate (RRa™) that is accurate, easy-to-use, and enhances patient compliance. Masimo Rainbow SET Acoustic Monitoring may enable earlier detection of respiratory compromise and patient distress—offering a breakthrough in patient safety for post-surgical patients on the general floor. Comments received from respiratory therapists at the AARC Congress included the following:
David Gibson, RRT, Supervisor, Medical City Dallas Hospital in Dallas, Texas, stated: "As a caregiver, I appreciate Masimo's commitment to innovation—they have done it again with their new Acoustic Respiration Rate Monitoring technology. Finally, there is a reliable, easy, comfortable, and cost-effective way to monitor breathing sounds on my patients on the general floor."
Jenni Raake, MBA, RRT, Respiratory Care Coordinator, Cincinnati Children's Hospital Medical Center, stated: "I see so many uses for Masimo's Rainbow Acoustic Monitoring, across so many care areas. With the wireless Masimo Rad-87 monitor, I believe it will be a great technology for patients needing ambulation. It should also be perfect for conscious sedation procedures."
Abbie Rosenberg, RRT, RCP in Santa Cruz, California, added: "I believe that Masimo Acoustic Respiration Rate will help hospitals effectively monitor and avoid sentinel events in post-surgical patients using patient controlled analgesic pumps."
Joe Kiani, Founder & CEO of Masimo, stated: "We are honored to receive the Zenith Award. This recognition is especially gratifying because it comes from the clinicians who use our products every day. It's also exciting to see the reactions and hear the positive reviews from respiratory therapists who saw first-hand demonstrations of Rainbow Acoustic Monitoring."
Michael O'Reilly, MD, Masimo's EVP of Medical Affairs, stated: "Respiratory care professionals attending the AARC Congress were truly excited by the introduction of Rainbow SET Acoustic Monitoring with its ability to monitor the breathing status of patients, 24/7. Respiratory care professionals are not alone in their excitement, as all clinicians who care for patients receiving patient-controlled analgesia for pain management know that sedation can slow breathing and place patients at considerable risk. For these patients, early intervention and appropriate treatment could mean the difference between life and death."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and acoustic respiration rate (RRa), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the breakthrough respiration rate monitoring capabilities of Masimo Rainbow SET Acoustic Monitoring technology will allow clinicians to detect and treat respiratory compromise and patient distress earlier, risks related to our belief that Masimo Rainbow SET Acoustic Monitoring technology will provide sufficient sensitivity and specificity to measure respiratory rate in a variety of patients and monitoring conditions, risks related to our assumptions regarding the commercial timing and availability of the technology, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Brookdale University Hospital and Medical Center Completes System-wide Conversion to Masimo SET® Pulse Oximetry and Adds Noninvasive Hemoglobin to Select Care Areas
Standardization Makes Large Teaching Hospital in Brooklyn First in New York to Leverage Noninvasive and Continuous Hemoglobin for Bloodless Hemoglobin Monitoring
Irvine, California – December 7, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that Brookdale University Hospital and Medical Center in Brooklyn, New York, has completed its system-wide conversion to Masimo SET pulse oximetry technology. As part of its system-wide technology standardization, Brookdale has also installed first-ever continuous and noninvasive hemoglobin monitoring (SpHb™) technology in select care settings—allowing clinicians to detect and treat chronic or acute anemia earlier, identify internal bleeding sooner, and more effectively manage blood transfusions for their patients.
By converting to Masimo SET, Brookdale clinicians gain all the Measure-Through Motion and Low Perfusion benefits of Masimo SET pulse oximetry, along with the breakthrough noninvasive blood constituent monitoring capabilities of Masimo Rainbow SET Pulse CO-Oximetry. Masimo Rainbow SET is the first-and-only technology platform capable of continuously and noninvasively measuring multiple blood constituents that previously required invasive procedures, including: total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI). The ability to immediately detect and treat potentially life-threatening conditions—without having to draw blood and wait for the results—enables earlier and better clinical decisions that may help save lives and improve patient outcomes.
According to Thomas Crimi, M.D., Director of the Blood Conservation Program at Brookdale, Masimo SpHb is helping to improve blood management and conservation at Brookdale. "SpHb provides us with the unique ability to proactively identify and address patient's requiring blood transfusions much earlier, while avoiding unnecessary transfusions and other invasive tests. This certainly contributes to better quality care, patient safety, and improved clinical outcomes."
Masimo Founder and CEO, Joe E. Kiani, stated, "Brookdale's history of implementing sophisticated diagnostics and treatment technologies makes it the ideal care setting to pioneer Masimo SpHb for Brooklyn-area residents. We applaud their use of advanced medical technologies to help improve blood conservation efforts, patient care and clinical outcomes within their community."
The Brookdale University Hospital and Medical Center is one of Brooklyn's largest voluntary nonprofit teaching hospitals with 530 inpatient beds. With an extensive ambulatory care network, and an Emergency Department and Level I Trauma Center that is among the busiest in the region, Brookdale is also a New York State DOH designated Stroke Center.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, and acoustic respiration rate (RRa), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SET and Masimo Rainbow SET will provide sufficient sensitivity and specificity to detect physiological abnormalities in real-time, enabling clinicians to detect and identify potentially life-threatening conditions earlier and make better clinical and treatment decisions for their patients, risks related to our assumption that Masimo SET and Masimo Rainbow SET will deliver a sufficient level of clinical improvement over alternative pulse oximetry and noninvasive patient monitoring technologies to allow for further adoption of the technology at other hospitals, and risks related to our assumption that the conversion of Brookdale University Medical Center will serve to substantially increase revenues, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Ruth Richman
Brookdale University Hospital
(718) 240-5345
rrichman@brookdale.edu
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Receives FDA Clearance for Masimo Rainbow SET® Acoustic Respiration Rate Monitoring
The First Acoustic Respiration Rate Monitoring Technology will Debut at the Annual American Association of Respiratory Care Conference
Irvine, California – December 1, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced FDA clearance of its latest innovation—Masimo Rainbow SET Acoustic Monitoring™—providing noninvasive and continuous respiration rate (RRa™) that is accurate, easy-to-use, and enhances patient compliance. Masimo Rainbow SET Acoustic Monitoring may enable earlier detection of respiratory compromise and patient distress – offering a breakthrough in patient safety for post-surgical patients on the general floor. The limited market release, which will begin in December, will allow select hospitals to be the first to benefit from the new technology.
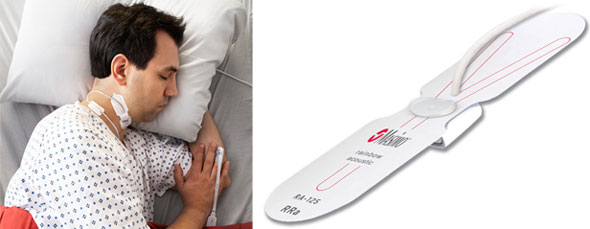
Masimo Rainbow SET Acoustic Monitoring noninvasively and continuously measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck to detect upper airway acoustic vibrations on the surface of the skin during the respiratory cycle.
Respiration rate is defined as the number of breaths per minute and is considered a critical vital sign in assessing the physiological status of hospitalized patients. Continuous monitoring of respiration rate is especially important for post-surgical patients receiving patient-controlled analgesia (PCA) for pain management as sedation can induce respiratory depression and place patients at considerable risk of serious injury or death.1-4 Although the Anesthesia Patient Safety Foundation (APSF) recommends continuous oxygenation and ventilation monitoring in all patients receiving opioids,5,6 current methods for respiration rate monitoring are limited by accuracy and patient compliance.
Masimo Rainbow SET Acoustic Monitoring features an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck to detect upper airway acoustic vibrations on the surface of the skin during the respiratory cycle. Using patented acoustic signal processing that leverages Masimo's revolutionary Signal Extraction Technology (SET), the respiratory signal is separated and processed to display continuous respiration rate.
When Masimo Rainbow SET Acoustic Monitoring is used in conjunction with Masimo Rainbow SET Pulse CO-Oximetry and the Masimo Patient SafetyNet™ Remote Monitoring and Clinician Notification System, clinicians can follow key indicators of oxygenation with Masimo SpO2, ventilation with the breakthrough RRa, circulation with Masimo measure through motion pulse rate (PR), and bleeding with Masimo noninvasive and continuous hemoglobin (SpHb™) – enabling them to monitor more patients, more safely, than ever before.
According to Michael Ramsay, M.D., Chief of the Department of Anesthesiology and Pain Management at Baylor University Medical Center in Dallas, Texas, "Breathing adequately is what matters most. Masimo acoustic respiration rate provides clinicians with the ability to automatically and continuously monitor the breathing status of post-surgical patients in general care or post-anesthesia settings—alerting them to the first sign of an abnormal or compromised breathing pattern that may be indicative of airway obstruction or respiratory distress." After using the new technology, Dr. Ramsay also stated "The Masimo Acoustic Sensor is virtually unnoticeable when worn – making it extremely patient-friendly."
Masimo Founder and CEO, Joe E. Kiani, stated, "Solving 'unsolvable' problems that have plagued patient monitoring is key to advancing medicine and improving patient care. Masimo Rainbow SET Acoustic Monitoring addresses a longstanding and growing need to accurately and easily monitor patient breathing, which is expected to improve patient safety and decrease the cost of care in hospitals. It also adds another compelling reason for hospitals to choose the Masimo Rainbow SET upgradeable platform for pulse oximetry, Pulse CO-Oximetry, and now—Acoustic Monitoring."
Live demonstrations of Masimo Rainbow SET Acoustic Monitoring will be featured at the Masimo exhibit at the American Association of Respiratory Care December 5-7, 2009 in San Antonio, Texas.
1 Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Alert: Patient controlled analgesia by proxy. Chicago: JCAHO, 2004
2 Institute for Safe Medication Practice. Safety issues with patient-controlled analgesia: Part 1-How errors occur. Huntingdon Valley: ISMP, 2003
3 Institute for Safe Medication Practices. Safety issues with patient-controlled analgesia: Part II – How to prevent errors Huntingdon: ISMP, 2003
4 Bird M. Acute pain management: a new area of liability for anesthesiologists ASA Newsletter. Park Ridge: American Society of Anesthesiologists, 2007
5 Weinger MB. Dangers of Postoperative Opioids: APSF Workshop and White paper address prevention of postoperative respiratory complications. APSF Newsletter, 2006;21:61–7. http://www.apsf.org/newsletters/html/2007/winter/01_opioids.htm
6 Stoelting RK, Weinger MB. Dangers of Postoperative Opioids—Is There a Cure? APSF Newsletter, 2009;24:25-26. http://www.apsf.org/newsletters/html/2009/summer/01_opiods.htm
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI™, and acoustic respiration rate (RRa), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results and performance of Masimo Rainbow SET Acoustic Monitoring, risks related to our belief that the breakthrough respiration rate monitoring capabilities of Masimo Rainbow SET Acoustic Monitoring technology will allow clinicians to detect and treat respiratory compromise and patient distress earlier, risks related to our belief that Masimo Rainbow SET Acoustic Monitoring technology will provide sufficient sensitivity and specificity to measure respiratory rate in a variety of patients and monitoring conditions, risks related to our assumptions regarding the commercial timing and availability of the technology, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, RRa, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Advocate Health Care Renews Multi-Year, System-wide Contract for Masimo SET® Pulse Oximetry Technology
Irvine, California – November 23, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that Advocate Health Care—Illinois' largest health care provider—has renewed its multi-year, system-wide contract for Masimo SET pulse oximetry technology. A Masimo customer since 2001, Advocate Health Care's renewal of its Masimo SET technology standardization contract means Masimo will continue to support the pulse oximetry needs of more than 200 Advocate sites of care across metropolitan Chicago.
Masimo SET pulse oximetry technology allows hospitals and other care sites to leverage the superior Measure-Through Motion and Low-Perfusion benefits of Masimo SET pulse oximetry, along with the unique breakthrough noninvasive blood constituent monitoring capabilities of Masimo Rainbow SET Pulse CO-Oximetry. Masimo Rainbow SET is the first-and-only technology platform capable of continuously and noninvasively measuring multiple blood constituents that previously required invasive procedures, including: total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI). The ability to immediately detect and treat potentially life-threatening conditions—without having to draw blood and wait for the results—enables earlier and better clinical decisions that may help save lives and improve patient outcomes.
"Advocate Health Care's healing mission calls us to work with partners that share our commitment to our patients' health and safety," said Paul Hoffmann, R.R.T., chairman of the Advocate Health Care respiratory care council. "We are very satisfied with the features and functionality of the Masimo pulse oximetry technology, and we are pleased to continue our relationship with Masimo."
Masimo Founder and CEO, Joe E. Kiani, stated, "Advocate Health is one of the premier health care institutions in the US. We are proud to have earned Advocate's trust over the last eight years and that they have decided to place their trust, business, and pulse oximetry needs in our hands for many more years."
Advocate Health Care, one of the nation's top 10 health systems based on clinical performance, is the largest health care provider in Illinois. As a not-for-profit, mission-based health system, Advocate operates more than 200 sites of care across metropolitan Chicago, including nine acute care hospitals, two children's hospitals, four Level I trauma centers (the state's highest designation in trauma care), a home health care company and one of the region's largest medical groups.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SET and Masimo Rainbow SET will provide sufficient sensitivity and specificity to detect physiological abnormalities in real-time, enabling clinicians to detect and identify potentially life-threatening conditions earlier and make better clinical and treatment decisions that may help save lives and improve outcomes for their patients, risks related to our assumption that Masimo SET and Masimo Rainbow SET will deliver a sufficient level of clinical improvement over alternative pulse oximetry and noninvasive patient monitoring technologies to allow for further adoption of the technology at other hospitals, risks related to our assumption that the system-wide renewal of Advocate Health Care will serve to substantially increase revenues, and risks related to our assumptions regarding the future of the Advocate Health Care system-wide pulse oximetry renewal contract, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Kelly Jo Golson
Adovate Health Care
(630) 990-5615
kellyjo.golson@advocatehealth.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at the 21st Annual Piper Jaffray Health Care Conference
IRVINE, Calif., November 20, 2009 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced that its management is scheduled to present at the 21st Annual Piper Jaffray Health Care Conference at the New York Palace Hotel in New York on Tuesday, December 1, 2009, at 12:00 p.m. ET. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET® provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at http://www.masimo.com.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Uni.H.A. in France Signs Purchasing Agreement for Masimo SET® Pulse Oximetry Products
Network of 53 Hospitals Gain Access to Masimo SET Pulse Oximeters
Irvine, California – November 18, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that Lyon, France-based Uni.H.A., one of the largest healthcare Group Purchasing Organizations (GPO) in France, has signed a multi-year purchasing agreement for Masimo SET® pulse oximeters. The agreement offers preferred contract pricing to Uni.H.A.'s 53 member hospitals and provides for more than 1,000 Masimo SET bedside pulse oximeters and 650 handheld pulse oximeters to be purchased within four-years.
Masimo pulse oximeters feature the most advanced pulse oximetry technology available to deliver accurate, reliable measurements and meaningful alarms that reflect a patient's true oxygenation status. The superior fidelity of Masimo SET pulse oximetry technology—clinically-proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 and pulse rate measurements, even under the most difficult clinical conditions of patient motion and low peripheral perfusion—allows clinicians to maximize their efficiency by concentrating on caring for their patients, rather than chasing false alarms.
In addition, the agreement provides Uni.H.A.-member hospitals with an upgrade path to the breakthrough noninvasive blood constituent monitoring capabilities available only as part of the Masimo Rainbow SET Pulse CO-Oximetry technology platform. These unique noninvasive measurements—including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™ for fluid responsiveness—can be field-installed on Masimo Rainbow-ready devices through a simple software upgrade, allowing hospitals to easily purchase and add them when they are ready. Masimo Rainbow SET measurements enable earlier detection of potentially life-threatening conditions to help increase patient safety, improve the quality of care, and reduce the cost of care.
Jon Coleman, President of Masimo International, stated: "This agreement with Uni.H.A. provides many of Europe's most demanding hospitals with direct and open access to Masimo pulse oximetry and noninvasive blood constituent monitoring solutions that facilitate faster, easier and safer health decisions. We are happy to partner with Uni.H.A. in providing their member hospitals with the industry-leading medical technology solutions they need to advance patient care and outcomes."
Uni.H.A. members include 32 Hospital University Centers and 21 major hospitals across Europe that represent 45% of French state hospital expenditures. Today, Masimo SET pulse oximetry technology is at work in more than 100 top-ranked hospitals throughout France, including the largest public hospital system in Europe—Assistance Publique Hopitaux de Paris (AP-HP).
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our assumptions that a sole-supplier agreement with UNI.H.A. will expand European market access to Masimo SET pulse oximeters, risks related to our belief that Masimo SET will deliver sufficient sensitivity and specificity to deliver the most accurate and reliable measurements under all conditions of patient motion and low peripheral perfusion, and risks related to our assumption that this multi-year supplier agreement will serve to broaden the use of Masimo SET pulse oximetry or will serve to provide substantially increased revenues for the company, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


CareFusion Signs Multi-Year Technology Licensing Agreement for Masimo Rainbow SET® Pulse CO-Oximetry™
CareFusion plans to Integrate Breakthrough Noninvasive Blood Constituent Monitoring Capabilities into Future Infusion and Respiratory Products
Irvine, California – November 12, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, and CareFusion (NYSE: CFN), a leading global medical device company, today announced a multi-year technology licensing agreement establishing the breakthrough noninvasive blood constituent monitoring capabilities of Masimo Rainbow SET Pulse CO-Oximetry technology to be used in CareFusion infusion and respiratory products under development*.
Incorporating Masimo Rainbow SET Pulse CO-Oximetry technology will enable CareFusion's future infusion and respiratory products (under development*) to noninvasively and continuously measure multiple blood constituents to more accurately assess dyshemoglobins affecting oxygenation, predict fluid responsiveness, and provide earlier identification of respiratory compromise. The ability to automatically assess total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI® for fluid responsiveness, in addition to oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate directly from CareFusion's ventilators and infusion pumps may facilitate earlier detection, diagnosis and treatment of potentially life-threatening conditions.
Currently, CareFusion uses Masimo technology in the Alaris® SpO2 module to help safeguard PCA delivery through the Alaris® System. The Alaris® PCA module together with the Alaris® SpO2 module—featuring Masimo SET® pulse oximetry technology for accurate and trustworthy measure-through motion and low-perfusion SpO2, pulse rate, and perfusion index measurements—provides an additional safety net for risk factors not covered by programming safety and bar coding, such as previously undiagnosed clinical conditions and PCA by proxy. The system offers the clinician the unique ability to automatically pause PCA infusion if a patient falls below hospital-defined respiratory monitoring limits.
"Masimo is a great partner with excellent technology," said Gordon LaFortune, senior vice president and general manager of Infusion Systems for CareFusion. "We're happy to be working with them to incorporate their technology in our products."
Masimo President of OEM Business, Rick Fishel stated, "We look forward to a growing technology partnership with CareFusion and the possibilities created by the integration of Masimo Rainbow SET into CareFusion's infusion and respiratory products."
*Future vision: As of the date of the communication, the products under development have not been cleared for commercial sale by the FDA. The products under development may not be cleared for commercial sale by the FDA. The company may not make the products under development available for commercial sale.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to the timing, commercial availability, and regulatory clearance of CareFusion infusion and respiratory products under development that may or may not incorporate Masimo Rainbow SET, risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that the noninvasive blood constituent monitoring capabilities of Masimo Rainbow SET may facilitate earlier detection, diagnosis, and treatment of potentially life-threatening conditions to increase patient safety, improve the quality of care, and reduce the cost of care, risks related to our belief that Masimo SET will deliver sufficient sensitivity and specificity to deliver the most accurate and reliable measurements under all conditions of patient motion and low peripheral perfusion, and risks related to our assumption that Masimo SET will help improve the regulation and delivery of oxygen therapy to neonates, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at the Lazard Capital Markets 6th Annual Healthcare Conference
IRVINE, Calif., November 10, 2009 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced that its management is scheduled to present at the Lazard Capital Markets 6th Annual Healthcare Conference at The St. Regis Hotel in New York on Tuesday, November 17, 2009, at 10:25 a.m. ET. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET® provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at http://www.masimo.com.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Published Study Shows Masimo Rainbow SET® Pulse CO-Oximetry™ Reliably Determines Carbon Monoxide Levels in the Blood
Researchers Highlight the Unique Advantages of Masimo SpCO® to Facilitate Early Diagnosis of Carbon Monoxide Poisoning On-the-Scene
Irvine, California – November 5, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that a new peer-reviewed clinical study published in this month's Inhalation Toxicology demonstrates that Masimo Rainbow SET® Pulse CO-Oximetry (SpCO) provides reliable measurements of carbon monoxide (CO) in the blood that facilitate fast, accurate diagnosis of CO poisoning in pre-hospital emergency and rescue environments.1
Carbon monoxide – often called the silent killer - is a colorless, odorless, tasteless, and deadly gas that is extremely difficult to detect. Prior to Masimo Rainbow SET Pulse CO-Oximetry, an invasive blood draw followed by laboratory blood gas analysis was the only reliable method for diagnosing CO poisoning. Without immediate access to complex and costly laboratory blood analyzers to measure CO in the blood (COHb), emergency first responders were at a critical disadvantage—unable to confirm the diagnosis of CO poisoning necessary to initiate appropriate, often lifesaving treatment. Today, the portable, handheld Masimo Rad-57 Pulse CO-Oximeter provides an accurate and noninvasive way to detect elevated CO levels in the bloodstream in just seconds—allowing emergency first responders to quickly and easily detect CO poisoning on-the-scene and initiate prompt, possibly lifesaving treatment.
Dr. Piatkowski and colleagues at the University Hospital Aachen (Germany) used the Masimo Rad-57 Pulse CO-Oximeter to measure SpCO in 20 patients suspected of CO poisoning who were admitted to the burn center and in five healthy volunteers (used as a control group). Comparing SpCO measurements to COHb measurements obtained hourly via invasive blood samples processed using three different laboratory analyzers, researchers found that the mean error of the three different blood gas analyzers was just 1% lower than noninvasive Pulse CO-Oximetry. The SpCO measurements from Pulse CO-Oximetry resulted in a mean error of approximately 3.15% over a COHb range of 1% to 38%, while the invasive COHb measurements from three different blood analyzers resulted in a mean error of 2.4%. Researchers concluded that Pulse CO-Oximetry "represents a reliable measurement technique that is easy to handle and could facilitate the early diagnosis of CO intoxication in pre-hospital rescue conditions."
Masimo Executive Vice President of Medical Affairs, Dr. Michael O'Reilly, stated, "This study adds to the compelling evidence base showing that Masimo Pulse CO-Oximetry SpCO provides accurate and reliable measurements that enable clinicians and first responders to make earlier and better decisions. This study is also the first to show that the differences in COHb measurement from different laboratory analyzers are similar to the differences between laboratory analyzer measurement and noninvasive Pulse CO-Oximetry measurement."
1 A. Piatkowski, D. Ulrich, G. Grieb, N. Pallua. "A New Tool for the Early Diagnosis of Carbon Monoxide Intoxication." Inhalation Toxicology, November 2009; 21(3):1144-1147.
*Editor's Note: The study is available online at:
http://informahealthcare.com/doi/abs/10.3109/08958370902839754
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo Rainbow SET will provide sufficient sensitivity and specificity to deliver the most accurate and reliable SpCO measurements under all emergency and rescue conditions, and risks related to our belief that the Rad-57 enables fast, reliable, accurate diagnosis of carbon monoxide poisoning, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks,
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports Third Quarter 2009 Financial Results
Q3 2009 Highlights:
- Product revenues increased 14% to $75.1 million from $66.1 million in prior year
- Rainbow revenues increased approximately 100% to $6.0 million from $3.0 million in prior year
- Shipped 26,200 Masimo SET and Masimo Rainbow SET pulse oximetry units
Livewire – Irvine, California, November 3, 2009 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced its financial results for the third quarter of 2009.
For the third quarter of 2009, Masimo reported product revenues of $75.1 million representing a 14% increase over $66.1 million for the third quarter of 2008. Including royalty revenues, Masimo reported total 2009 third quarter revenues of $87.4 million compared to $78.1 million for the third quarter of 2008. For the third quarter of 2009, Masimo reported earnings per share of $0.22 compared to $0.22 per share for the third quarter of 2008.
Masimo reported that it shipped 26,200 Masimo SET and Masimo Rainbow SET pulse oximetry units, excluding handheld units, during the third quarter of 2009, resulting in over 600,000 Masimo SET and Masimo Rainbow SET pulse oximeters in use worldwide. In the third quarter of 2009, revenues from Masimo Rainbow SET products increased to $6.0 million from $3.0 million in the same prior year quarter.
For the first nine months of 2009, Masimo reported product revenues of $219.7 million representing a 17% increase over $188.0 million for the first nine months of 2008. Including royalty revenues, Masimo reported total 2009 year to date revenues of $256.5 million compared to $224.0 million for the prior year period. For the first nine months of 2009, Masimo reported earnings per share of $0.65, up 20% from $0.54 per share for the first nine months of 2008.
Joe E. Kiani, Chairman and Chief Executive Officer of Masimo, said, "We are encouraged by the continuing growth of our business, especially during a period of historical economic turmoil. We are seeing great enthusiasm combined with solid growth for our latest innovation, Masimo Rainbow SET technology, which has ushered in new noninvasive measurements, including hemoglobin and carbon monoxide."
Masimo also reported that as of October 3, 2009, cash and cash equivalents totaled $174.7 million, up from $146.9 million at January 3, 2009.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation number for both dial-in numbers is 35275093. A live web cast of the conference call will be available online from the "Investor Relations" page of the Company's corporate website at www.masimo.com. After the live web cast, the call will remain available on Masimo's website through December 3, 2009. In addition, a telephonic replay of the call will be available until November 17, 2009. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 35275093.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com. Information on the company's website does not form part of this press release.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations, prospects and business generally; and expectations regarding our ability to design and deliver innovative new noninvasive technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo Rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any new products and technologies of ours; obtaining regulatory approval of our current and future products and technologies, including the recently announced total hemoglobin measurement; the risk that the implementation of our international realignment, will not produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.

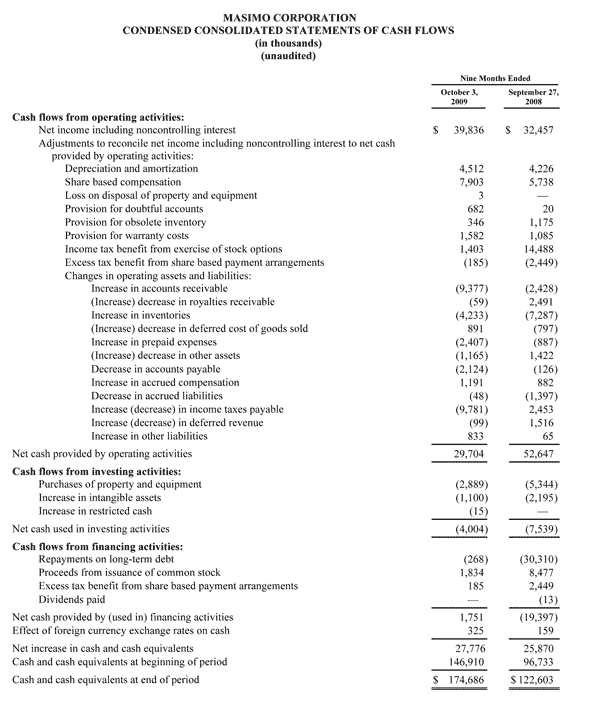


Federal Court of Appeals Upholds Antitrust Liability Verdict Against Tyco HealthCare
Affirms 2006 District Court ruling that found Tyco unlawfully maintained monopoly power and utilized unlawful restraints of trade and exclusionary dealing arrangements in the pulse oximetry market
Irvine, California – November 2, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that the Ninth Circuit Court of Appeals has affirmed the 2006 Federal District Court decision that Tyco Healthcare, now Covidien, violated the antitrust laws through anticompetitive business practices related to the sale of its Nellcor pulse oximetry products. The 2006 decision found that Tyco had unlawfully maintained monopoly power in violation of Section 2 of the Sherman Act, and that Tyco's sole-source agreements and market-share based compliance pricing contracts were unlawful restraints of trade in violation of Section 1 of the Sherman Act and unlawful exclusionary dealing arrangements in violation of Section 3 of the Clayton Act. The Ninth Circuit also held that above-cost bundling discounts when combined with sole-source or market-share-based pricing can be anticompetitive when such practices involve a significant portion of the market. The suit was originally filed by Masimo in 2002.
Joe E. Kiani, Founder and CEO of Masimo, stated: "We hope that the results that we have fought for will help improve patient care and reduce the cost of care by improving caregivers' access to cost-effective, innovative products. Medical products, from drugs and implantable devices to pulse oximeters, should be judged on their own merits and not based on artificial restraints on hospital purchasing placed by large manufacturers. America is struggling with rising health care costs. The best medicine for this challenge is innovation and competition. Since Masimo began our travails in the Senate and the courts to restore competition, American hospitals and care givers have begun to have more choice. That choice in pulse oximetry has not only caused pulse oximetry pricing to decrease by over 30%, but many people's lives were either saved or improved as a direct result of their access to Masimo pulse oximeters. We hope this ruling will improve the accessibility of all life-saving products for the best care at lowest price possible for patients."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo Rainbow SET and Masimo PVI provide unique clinical advantages that facilitate earlier intervention and prevention of adverse patient outcomes, risks related to our assumptions that Masimo SpHb is an accurate, reliable and acceptable clinical alternative to invasive blood tests for hospitalized patients and healthy patients in outpatient settings, and risks related to our assumptions that Masimo PVI is an accurate, reliable and acceptable clinical alternative to invasive methods of predicting fluid responsiveness, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mark de Raad
Masimo Corporation
(949) 297-7041
mderaad@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.

Masimo® Inaugurates International Operations Center in Switzerland
New International Headquarters Part of Masimo's Commitment to Support and Serve the Global Medical Community
Livewire (Internal Only) - Irvine, California – October 27, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today the inauguration of its international operations center in Neuchatel, Switzerland. Masimo International SARL was initially established in December 2008 to serve as Masimo's international headquarters. Today's formal inauguration ceremony at the Neuchatel facility underscores the company's commitment to growing and expanding its operations to better serve the international medical community.
Masimo Founder and CEO, Joe E. Kiani, stated, "Neuchatel is a growing, thriving center for medical technology companies that offers the best of Switzerland—from its excellent infrastructure and direct access to the European markets to its favorable business climate. This affords us the opportunity to build a world class international team and business structure to continue to grow our business."
"Masimo is an important addition that will not only add to the creation of jobs and economic investment, but will also further enhance Neuchatel's position as a growing center for medical technology," stated Frederic Hainard, State Counselor in charge of the Department of the Economy for the Canton of Neuchatel, Switzerland. "This reinforces the attractiveness of the Canton of Neuchatel and its international influence as a favorable residence for growing corporations. We are pleased to welcome Masimo and look forward to their continued growth and success in the Canton of Neuchatel."
Masimo—a global developer and manufacturer of innovative noninvasive patient monitoring technologies that significantly improve patient care—will centralize its international sales management, marketing, planning, logistics, customer service, technical support, finance and administrative activities at the Neuchatel facility to better and more responsively serve international customers. As the company continues to expand to meet the growing global demand for its breakthrough medical technologies and products, its international operations will enable direct sales and service channels for Masimo medical technologies and products in international markets, facilitate direct support and expand interaction between the company and clinicians throughout Europe, Asia Pacific, Africa, the Middle East, Canada, and Latin America.
According to Jon Coleman, President of International Business for Masimo, "Growing international demand for Masimo's advanced medical technologies and solutions is the driving force behind the establishment of our new international operations. Staffed with dedicated resources in key business areas—all focused exclusively on servicing our growing international customer base—the Neuchatel facility will be the nerve center of our international operations. Ultimately, this will ensure that we are more proactively and aggressively working to meet the needs of medical providers in international markets."
Masimo's new international operations center is expected to contribute new technical and professional jobs and investment in local infrastructure projects and subcontract activities to the local economic profile of the region. Today's inauguration was hosted by Neuchatel's Economic Promotion Office and was attended by Neuchatel's State Counselor Monsieur Frederic Hainard, key Masimo executives, and European customers.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the new International operations in Neuchatal will contribute or create new jobs, subcontract activities, new opportunities for R&D collaboration, and synergistic relationships between medical technology and biotechnology, and risks related to our belief that the new headquarters will operations enable the company to better and more responsively serve international customers, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Federic Hainard
Department of the Economy – Canton of Neuchatel
+ 41 32 889 68 00
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Clinical Studies Demonstrate the Accuracy of Masimo Noninvasive and Continuous Hemoglobin Monitoring Technology
Irvine, California – October 21, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that new clinical studies presented this week at the American Society of Anesthesiology (ASA) Annual Meeting in New Orleans, Louisiana, show Masimo noninvasive and continuous hemoglobin (SpHb™) technology is reliable and accurate compared to invasive blood tests for both healthy subjects and hospitalized patients. Another study presented found Masimo PVI to be an accurate, reliable automatic noninvasive indicator of a patient's ability to respond to fluid.
Clinical Study Highlights
Accuracy of Noninvasive Hemoglobin Measurements by Pulse CO-Oximetry in Hemodilution Subjects (A184)1, a clinical study led by Dr. Martin W. Allard at Loma Linda University in Loma Linda, California, compared SpHb measurements to 165 invasive laboratory measurements in 20 healthy adult patients undergoing hemodilution. All 20 patients had one unit of blood drawn through an arterial line while isolyte intravenous fluid was given (to compensate for the decrease in intravascular volume) until they reached a 30% reduction in hemoglobin or a maximum of 30 ml/kg of fluid. Blood samples were drawn after each 500 ml of fluid administered and analyzed for total hemoglobin by laboratory CO-Oximeter. Masimo SpHb had a bias of 0.15 g/dL and precision of 0.92 g/dL, leading researchers to conclude that SpHb "measurement accuracy was unaffected by perfusion index levels" and offers an "acceptable alternative to invasive hemoglobin tests in many clinical scenarios."
Validation of a New Noninvasive Hemoglobin Algorithm in Patients Undergoing Liver Transplantation (A751)2, a clinical study led by Dr. Klaus D. Torp at the Mayo Clinic in Jacksonville, Florida, compared SpHb measurements to 55 invasive laboratory measurements in five patients undergoing liver transplantation and found significant agreement between the two methods. The study showed that SpHb—using the new Masimo ReSposable™ Sensor that helps to reduce medical waste and costs——had a bias of 0.2 g/dL and precision of 0.8 g/dL. Researchers concluded that "the accuracy of noninvasive SpHb measurements obtained by the Pulse CO-Oximeter with new ReSposable sensors was high."
Additionally, two studies presented at the ASA highlight the importance of understanding the variation in invasive arterial and venous hemoglobin measurements. A study (A937) of 471 paired invasive hemoglobin measurements from 33 patients on two different invasive laboratory devices (Beckman Coulter® hematology analyzer and Nova Biomedical® CO-Oximeter) showed a bias of -0.97 g/dL and a precision of 0.58 g/dL. The authors noted that "different devices using different principles of operation can produce consistent differences in the absolute value of measurement".3 Another study (A1294) comparing hemoglobin measurements in 107 subjects from time-matched arterial and venous blood samples on the same invasive laboratory device found average differences as high as 0.5 g/dL and noted that "arterial and venous hemoglobin values from the same individual are not interchangeable."4
Influence of Site of Measurement to Predict Automatically and Noninvasively Fluid Responsiveness (A477)5, a clinical study led by Dr. Olivier Desebbe at the Hospices Civils de Lyon in Lyon, France, studied the finger, forehead, and ear as measurement sites for PVI and found that all three sites allowed discrimination between fluid responders and non-responders. Researchers measured hemodynamic data in 12 patients under general anesthesia using pulmonary artery catheter and Masimo PVI (obtained via noninvasive sensors attached to the forehead using a Masimo TF-I, ear using a Masimo TC-I, and finger using a Masimo LNOP Adt) before and after volume expansion of 500 ml of hetastarch 6%. Fluid responders were defined as patients presenting >15% increase in cardiac index (CI) following VE. Study results showed that a PVI >17% for the forehead and ear, and a PVI >13% for the finger before volume expansion "allowed discrimination between responders and non-responders with a sensitivity and specificity of 100 and 83% for the forehead, 83 and 100% for the ear, and 100 and 67% for the finger"—demonstrating that PVI is an accurate and reliable automatic noninvasive indicator of a patient's ability to respond to fluid and that clinicians should take site measurement into account when utilizing PVI to predict fluid responsiveness.
Dr. Michael O'Reilly, Executive Vice President of Medical Affairs for Masimo, stated: "We appreciate the work of these clinical researchers for not only validating the value of SpHb and PVI, but for also showing that invasive hemoglobin spot check measurements—considered to be the gold standard—have similar bias and precision as our noninvasive, continuous hemoglobin measurements. These new Masimo Rainbow SET studies reinforce the importance of monitoring changes in hemoglobin—facilitated by Masimo's continuous SpHb monitoring technology as opposed to a single measurement of hemoglobin—when caring for patients at risk for significant blood loss."
1 Allard, M., Macknet, M., Kherzi, S., Rook, J. "Accuracy of Noninvasive Hemoglobin Measurements by Pulse CO-Oximetry in Hemodilution Subjects." ASA 2009 Annual Meeting: A184. Anesthesiology, Loma Linda University, Loma Linda, California.
2 Torp, K., Aniskevich, S., Shine, T., Shapiro, D., Peiris, P. "Validation of a New Noninvasive Hemoglobin Algorithm in Patients Undergoing Liver Transplantation." ASA 2009 Annual Meeting: A751. Anesthesiology, Mayo Clinic Florida, Jacksonville, Florida.
3 Torp, K., Shine, T., Aniskevich, S., Peiris, P., Shapiro, D. "Comparison of Coulter Counter and Co-Oximeter (pHOx) Measurements for Hemoglobin." ASA 2009 Annual Meeting: A937. Anesthesiology, Mayo Clinic Florida, Jacksonville, Florida.
4 Rook, J., Khezri, S., Bertolizio, G., Allard, M. "Arterio-Venous Hemoglobin Differences in Healthy Subjects During Rapid Volume Expansion." ASA 2009 Annual Meeting: A1294. Department of Anesthesiology, Loma Linda University Medical Center, Loma Linda, California.
5 Desebbe, O., Robin, J., Lehot, J., Cannesson, M. "Influence of Site of Measurement to Predict Automatically and Noninvasively Fluid Responsiveness." ASA 2009 Annual Meeting: A477. Anesthesiology and Intensive Care, Hospices Civils de Lyon, Lyon Bron, France.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo Rainbow SET and Masimo PVI provide unique clinical advantages that facilitate earlier intervention and prevention of adverse patient outcomes, risks related to our assumptions that Masimo SpHb is an accurate, reliable and acceptable clinical alternative to invasive blood tests for hospitalized patients and healthy patients in outpatient settings, and risks related to our assumptions that Masimo PVI is an accurate, reliable and acceptable clinical alternative to invasive methods of predicting fluid responsiveness, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report Third Quarter 2009 Financial Results on November 3, 2009
Conference call and webcast to begin after markets close at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., October 20, 2009—Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced it will release third quarter 2009 financial results for the period ended October 3, 2009, after the market closes on Tuesday, November 3, 2009.
A conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe E. Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the Company's corporate web site at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation number for both dial-in numbers is 35275093. After the live webcast, the call will remain available on Masimo's website through December 3, 2009. In addition, a telephonic replay of the call will be available through November 17, 2009. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 35275093.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at http://www.masimo.com.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


The MED Group Signs Preferred Supplier Agreement for Masimo SET® Pulse Oximetry Solutions
Home Medical Equipment GPO Expands Access to Masimo SET Pulse Oximeters for Home Care Providers
Irvine, California – September 24, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, and The MED Group, a Group Purchasing Organization (GPO) for home care providers, jointly announce a preferred supplier agreement that will give MED Group members preferred contract pricing on Masimo SET Pulse Oximeters and sensors. Masimo's solutions are proven to help caregivers improve patient safety, reduce costs, and increase efficiencies.
The industry leader in pulse oximetry, Masimo SET is clinically-proven in more than 100 independent and objective studies to deliver the most accurate and reliable SpO2 and pulse rate measurements, even under the most challenging clinical conditions of patient motion and low peripheral perfusion In fact, research shows that the unmatched sensitivity and specificity of Masimo SET is not only more accurate in detecting true desaturation events—including brief dips in oxygen saturation as well as larger ones, but it can also "significantly reduce workload and improve reliability of desaturation detection" over other pulse oximeters.1
Wayne Grau, Vice President Supplier Relations, The MED Group, stated "Ensuring the continued success of our members is a top priority. Two of the best ways we accomplish this include providing access and preferred pricing on the high-quality products that help them take better care of their patients and reducing unnecessary costs in the home care environment. Masimo SET pulse oximetry products do both, by enabling home care clinicians to more accurately and reliably monitor their patients without having to waste time and resources sorting through false alarms."
Masimo SET products immediately available to MED Group members include the new Masimo Rad-8 pulse oximeter and the full compliment of Masimo SET adhesive and reusable sensors. The new Rad-8 offers a host of easy-to-use special features that help caregivers more effectively and efficiently capture, analyze, and report vital oxygen saturation, pulse rate, and perfusion index information for improved patient care in the home environment.
Masimo Founder and CEO, Joe E. Kiani, stated "Masimo has proudly served the needs of the home care market since the introduction of Masimo SET more than 10 years ago and we look forward to expanding our service in this market as a preferred pulse oximetry supplier to MED Group members. Our partnership with The MED Group will make Masimo SET pulse oximetry products available to their entire network of market-leading home medical equipment customers and home care providers to help advance the care and improve the safety of patients at home."
1 Robert Brouillette, MD, Jacinthe Lavergne, RRT, Andra Leimanis, BSc, Gillian Nixon, MB ChB, FRACP, Sylvia Ladan, RRT, Christine McGregor, RRT. "Differences in Pulse Oximetry Technology can Affect Detection of Sleep-Disordered Breathing in Children." Anesth Analg 2002; 94: S47-S53.
About The MED Group
The MED Group is a Group Purchasing, Business Solutions and Referral Management Network that serves its Home Care Customers (Members, Suppliers and Referral Sources) through a unique model that delivers value for each. MED Members are market-leading Home Medical Equipment (HME) Providers that consistently deliver quality patient care through clinical and business based best practices. Through MED smart purchasing economic models, members make cost effective purchasing choices for products available through MED Preferred Supplier agreements. MED also helps Members improve efficiencies in education and training, reimbursement, accreditation, certification, technology and other operational areas – to help maintain their status as market leaders. MED's Network includes 365+ market leading HME providers that cover 1000+ locations. MED's national headquarters are located in Texas. For more information about MED, visit: http://new.medgroup.com/.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with http://www.aarc.org/education/meetings/congress_09/he Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our assumptions that a preferred supplier agreement with MED Group will expand market access to Masimo SET pulse oximeters, risks related to our belief that Masimo SET will deliver sufficient sensitivity and specificity to deliver the most accurate and reliable measurements under all conditions of patient motion and low peripheral perfusion, and risks related to our assumption that entering into a multi-year supplier agreement with MED Group will serve to broaden the use of Masimo SET pulse oximetry or will serve to provide substantially increased revenues for the company, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


University Medical Center Mainz Completes Installation of Masimo Noninvasive and Continuous Hemoglobin Throughout their Operating Rooms, Intensive Care Units and Emergency Department
SpHb™ Technology Now Advancing Patient Care at One of Largest Hospitals in Germany
Irvine, California – September 22, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that the University Medical Center of the Johannes Gutenberg University in Mainz, Germany has completed its clinical installation of Masimo noninvasive and continuous hemoglobin (SpHb) technology in their ORs, ICUs and EDs, as part of their efforts to improve patient care.
According to clinicians at the University Medical Center Mainz, their 57 Masimo Radical-7® Pulse CO-Oximeters with continuous and noninvasive hemoglobin are already providing significant clinical advantages and patient care benefits by allowing them to more effectively manage blood transfusions, identify internal bleeding sooner, and detect and treat chronic or acute anemia earlier—all of which may help save lives and improve patient outcomes.
Christian Werner, M.D., Chair of the Department of Anesthesiology at the University Medical Center Mainz, stated, "Masimo noninvasive hemoglobin is an exciting novel technology with wide clinical applications, which may ultimately improve peri-operative outcome. Furthermore, noninvasive SpHb monitoring could also reduce overall treatment costs."
By choosing Masimo Radical-7 Pulse CO-Oximeters, Mainz clinicians have also gained all the benefits of Masimo SET Measure-Through Motion and Low-Perfusion pulse oximetry, proven superior in over 100 independent and objective clinical studies. Masimo Rainbow SET is the first and only technology platform capable of continuously and noninvasively measuring multiple blood constituents that previously required invasive procedures, including: hemoglobin (SpHb™), oxygen content (SpOC™) carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™ for fluid responsiveness, in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate.
Masimo Founder and CEO, Joe E. Kiani, stated, "This multi-department installation at a leading German hospital is a good example of the growing recognition and adoption of Masimo SpHb, which gives hospitals and clinicians the opportunity to advance clinical practice in a way that has the potential to transform healthcare delivery. We applaud University Medical Center Mainz for recognizing the value of SpHb and for leading the advancement of patient care with the deployment of Masimo Rainbow SET across their ORs, ICUs and EDs."
The University Medical Center Mainz is a healthcare facility, as well as a research and teaching hospital that leverages state-of-the-art diagnostic and therapeutic procedures to optimize patient care. It consists of more than 50 specialist clinics, institutes and departments, as well as two central health-care facilities, a pharmacy and a transfusion center. The hospital has over 1,600 beds and treats approximately 61,000 in-patients and 217,000 out-patients every year.
About University Medical Center of the Johannes Gutenberg University Mainz
The 'University Medical Center Mainz' consists of more than 50 specialist clinics, institutes and departments as well as two central health-care facilities, a pharmacy and a transfusion center. All of these clinics and institutes work together on an interdisciplinary basis. The hospital has over 1600 beds and treats more than 61,000 in-patients every year. It also serves an additional 217,000 people per year on an out-patient basis.
We define ourselves as the patients' best source for optimum patient care. In our work, we use state-of-the-art diagnostic and therapeutic procedures based on recent medical research. It is our goal to serve as optimally as possible by integrating our knowledge and experience into the excellent services we provide. We offer nearly every kind of clinical facility you might need. The University Medical Center offers health care to the highest university standard. In addition, we are not only a health-care center; but also a research and teaching hospital. These three pillars of medicine – clinical health care, medical education and research – are inseparable aspects of integrated health care. For more information, please visit: www.unimedizin-mainz.de
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that the breakthrough blood constituent monitoring capabilities of Masimo Rainbow SET will allow clinicians to detect and treat life-threatening conditions earlier, risks related to our belief that Masimo noninvasive and continuous hemoglobin (SpHb) will provide sufficient sensitivity and specificity to instantaneously measure hemoglobin blood levels noninvasively and continuously to enable earlier identification of necessary blood transfusions, reduction in unnecessary transfusions and invasive tests, increased patient safety, and improved clinical outcomes, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


PHYSIO-CONTROL RECEIVES MARKET APPROVAL FROM HEALTH CANADA FOR LIFEPAK® 15 MONITOR/DEFIBRILLATOR
LIFEPAK 15 is the First Monitor/Defibrillator to Integrate Masimo Rainbow SET® Pulse CO-Oximetry™ Technology for the Noninvasive Detection of Carboxyhemoglobin and Methemoglobin
REDMOND, WASH. – Sept. 22, 2009 – Physio-Control, Inc., a wholly owned subsidiary of Medtronic, Inc., (NYSE: MDT) announced today it received approval by Health Canada to market the LIFEPAK 15 monitor/defibrillator within Canada. Official license notification was received on Sept. 1, 2009.
Physio-Control was granted CE mark in January 2009, certifying compliance with the European Union Medical Device Directive and began its U.S. market release of the LIFEPAK 15 monitor/defibrillator in March 2009.
The LIFEPAK 15 monitor/defibrillator builds on a 54-year Physio-Control heritage of providing innovative, reliable equipment to lifesaving teams around the world. With an all-new product platform, it delivers best-in-class clinical and operational innovations and raises the bar on the legendary LIFEPAK® standard for durability.
Clinically innovative, the LIFEPAK 15 provides the widest range of energy dosing up to 360 joules, and the broadest selection of monitoring options available. It continues Physio-Control's legacy of industry firsts as the first monitor/defibrillator to integrate Masimo Rainbow SET® Pulse CO-Oximetry™ technology for the noninvasive detection of carboxyhemoglobin (carbon monoxide in the blood; the leading cause of poisoning death in industrialized countries1) and methemoglobin in the blood (which may result from exposure to certain chemicals and drugs).
The design focuses on several key operational innovations which include the largest color screen on the market, with a one-touch button that flips the screen to high-contrast SunVue™ mode for easy viewing in sunlight; along with 10 times the processing speed and three times the battery life of its predecessor. Needed for the harshest rescue environments, Physio-Control developed the LIFEPAK 15 to be tougher than any other monitor/defibrillator on the market. For current LIFEPAK customers, this model reduces transition costs, with a user interface that complements the market-leading LIFEPAK 12 defibrillator/monitor. Emergency care providers are able to work with a mixed fleet of LIFEPAK monitors, and transition their equipment when budgets allow—enabling them to save on critical operational costs and minimize their overall training resources.
"Physio-Control has been developing and refining monitoring and defibrillation technology for more than 50 years. With the latest approval for distribution by Health Canada the LIFEPAK 15 continues to build momentum in the marketplace and is setting the new standard for emergency care products," said Brian Webster, president of Physio‐Control. "The '15' has already been put into use by multiple leading EMS systems around the world."
Masimo Founder and CEO, Joe E. Kiani, stated, "The LIFEPAK 15 monitor with Masimo Rainbow SET Pulse CO-Oximetry technology represents a significant advancement for emergency first responders who provide lifesaving care and treatment to combat a range of silent killers. The combination of a defibrillator with noninvasive carboxyhemoglobin (SpCO) and methemoglobin (SpMet) monitoring capabilities means that more cases of potentially life-threatening conditions may be detected and treated earlier to improve patient safety, outcomes and recoveries."
About Physio-Control
Physio-Control, a wholly-owned subsidiary of Medtronic, is located in Redmond, Wash. Physio-Control pioneered defibrillation technology more than 54 years ago. With nearly 800,000 LIFEPAK® defibrillators distributed worldwide, the company is the world's leading provider of external defibrillation and monitoring technology for the treatment of sudden cardiac arrest and other cardio-respiratory emergencies. To find out more about Physio-Control, go to www.physio-control.com or call 1-800-442-1142.
About Medtronic
Medtronic, Inc. (www.medtronic.com), headquartered in Minneapolis, is the global leader in medical technology – alleviating pain, restoring health, and extending life for millions of people around the world.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com. PR Contact: Dana Banks, Masimo Corporation, 949-297-7348.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's Annual Report on Form 10-K for the year ended April 24, 2009. Actual results may differ materially from anticipated results.
1 Omaye ST. (Nov 2002). "Metabolic modulation of carbon monoxide toxicity". Toxicology 180 (2): 139–50.
Contact:
Jeff Warren
Investor Relations
763-505-2696
Jennifer Roth
Media Relations
425-867-4393


Masimo Launches Enhanced Masimo Patient SafetyNet™ System to Help Hospitals Reduce Preventable Deaths on the General Floor
New Features Expand Monitoring Capabilities, Simplify Operation and Extend Capabilities to Cover Up to 80 Patients Per Server on Up to Four Floors
Irvine, California – September 15, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today introduced a new enhanced version of the Masimo Patient SafetyNet system designed to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue—one of today's most common medical errors.1 New features such as an intuitive touch-screen interface and the ability to monitor up to 80 patients simultaneously on four separate floors allow hospitals to noninvasively and remotely monitor more patients, in more care areas, more efficiently, and in more clinical detail—enabling an enhanced level of patient safety when a clinician can't be at the bedside.
The Masimo Patient SafetyNet remote monitoring and clinician notification system combines the performance of Masimo SET® pulse oximetry with optional Oridion Microstream® end tidal CO2-based respiration rate monitoring at the point of care and wireless clinician notification via pager to provide an unmatched level of patient safety on general care floors. The system uses IEEE industry standards for connectivity—allowing for more efficient sharing of data across a hospital's IT platforms and the option of full integration into a hospital's existing IT infrastructure, providing a lower overall cost of ownership and improved financial benefits.
One of the most common dangers in hospitals today is the risk of death or serious injury caused by medications designed to relieve pain and help keep patients comfortable. Growing concerns over the use of patient-controlled analgesia (PCA) in the post-operative period have caused leading patient safety organizations to institute new recommendations and guidelines to combat the "unexpected and potentially harmful opioid-induced respiratory depression that continues to occur." According to the latest direction from the Anesthesia Patient Safety Foundation (APSF), these dangers exist because "in most cases, there is inadequate monitoring of oxygenation and/or ventilation." The APSF further warns hospital clinicians that "intermittent subjective assessments of ventilation or level of consciousness are unreliable predictors of future respiratory depression, even over short time frames" and continuous monitoring of oxygenation via pulse oximetry must be "the routine and not the exception."2
The new Patient SafetyNet system is already having a big impact on both nurses and post-surgical patients for two hospitals involved in limited market release testing. Marilyn Nemerever, R.N., director of Acute Care at Swedish Medical Center in Seattle, where the new system is being used to monitor patients in three separate post-surgical units at three different hospitals from a single central monitoring station, stated "We love it. ICU beds are in high demand these days and Patient SafetyNet allows us to more closely monitor post-surgical patients in our med-surg units, so we can use our ICU resources more appropriately. Our nurses now have the piece of mind that comes with knowing that Patient SafetyNet is helping watch over their patients if and when they cannot. And our patients are having better outcomes because we can see, as well as respond to changes earlier."
Clinicians at Dartmouth-Hitchcock Medical Center, who found that Patient SafetyNet reduced rescue activations by 65% and ICU transfers by 48%—while in some patients where ICU transfer was avoided, length of stay was also reduced from 5.8 to 3.6 days with an associated cost of care reduction of 30%, have also embraced the capabilities of the new Patient SafetyNet system. George T. Blike, M.D., Medical Director of Patient Safety at Dartmouth-Hitchcock Medical Center in Lebanon, New Hampshire, stated; "The new system enhancements allow us to see real-time numerics for each patient at a glance, while the ability to monitor more patients on a single server will enable us to deploy the system across more care areas than before to reduce overall costs of implementation."
Another significant risk that post-surgical patients face is undetected internal bleeding. The Patient SafetyNet system can also be easily upgraded to Masimo Rainbow SET Pulse CO-Oximetry technology through a simple software upgrade—allowing hospitals to measure a patient's hemoglobin noninvasively and continuously, which may improve patient safety by providing an earlier indication of undetected internal bleeding and enabling immediate intervention.
Masimo Executive Vice President of Medical Affairs, Michael O'Reilly, MD, stated, "Each year healthy patients admitted for routine procedures become failure to rescue statistics because an adverse event was not recognized in time to save them. The new capabilities in Masimo Patient SafetyNet significantly expand the functionality of a system that has already been proven to help clinicians improve patient outcomes and decrease costs—two things every hospital wants and needs."
1 "The Sixth Annual HealthGrades Patient Safety in American Hospitals Study" April 2009.
http://www.healthgrades.com/media/DMS/pdf/PatientSafetyInAmericanHospitalsStudy2009.pdf
2 "Dangers of Postoperative Opioids—Is there a Cure?" APSF Newsletter: The Official Journal of the Anesthesia Patient Safety Foundation. Summer 2009; Volume 24, No. 2, 25-32.
http://www.healthgrades.com/media/DMS/pdf/PatientSafetyInAmericanHospitalsStudy2009.pdf
3 Dartmouth-Hitchcock Medical Center Clinical Abstract Presented at the Society for Technology in Anesthesia (STA) Annual Meeting in San Antonio, Texas on January 15, 2009.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Patient SafetyNet will provide an effective early warning system of a patient's deteriorating physiological condition to enable timely rescue, risks related to our belief that Masimo Rainbow SET Pulse CO-Oximetry measurements and Masimo Desat Index 3D Alarms will provide sufficient sensitivity and specificity to detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, risks related to our assumptions regarding the repeatability of Swedish Medical Center and/or Dartmouth-Hitchcock Medical Center clinical results, and risks related to our assumptions regarding the systems' ability to deliver clinical improvement over alternative patient monitoring and assessment methods to increase patient safety and allow for further adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks,
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


FDNY Makes Largest First Responder Purchase of Masimo Rad-57 Pulse CO-Oximeters to Improve Survival of Firefighters and Victims of Carbon Monoxide Poisoning
Nation's Largest Fire & EMS Provider Leads First Responder Deployments of Rad-57s Worldwide
Irvine, California – September 8, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced that the Fire Department of New York City (FDNY) has purchased 86 Masimo Rad-57 Pulse CO-Oximeters as part of their strategic initiative to "improve the survivability of firefighters and victims suffering from carbon monoxide (CO) toxicity." The purchase is the single largest first responder deployment of Rad-57s, making FDNY the largest department to implement a department-wide program for noninvasive carbon monoxide monitoring in the U.S.
The Masimo Rad-57 Pulse CO-Oximeter enables EMS and fire department first responders to quickly, accurately, and noninvasively detect carbon monoxide poisoning on the scene. FDNY will put its 86 new Rad-57s to work throughout the 5 boroughs of New York City on all 35 of its HazTac and rescue ambulances, Supervisor units, and Major Emergency Response Vehicles—allowing for prompt and possibly life-saving treatment for both victims and firefighters alike.
According to John Peruggia, Chief of EMS for the Fire Department City of New York, the Masimo Rad-57 Pulse CO-Oximeter has become a key component in FDNY fire and emergency medical response operations "because it allows our EMTs and Paramedics to accurately evaluate a patient's blood chemistry, determine toxicity levels, and commence initial treatment of patients found to be poisoned by CO—all within seconds. The Rad-57 has also become an integral part of firefighter rehab, with all fire personnel being evaluated for CO after operating at large fire incidents, to insure responder health and safety. Expanding our utilization of the Rad-57 with this purchase is a natural progression of the success we have achieved, both in terms of lives saved and hazards averted."
Masimo Founder and CEO, Joe E. Kiani, stated, "We commend FDNY for their leadership, dedication, and vigilance in the battle against the unsuspected, deadly hazards of carbon monoxide poisoning. We are particularly proud that they have chosen the Rad-57 to help ensure the public health and safety of all New York City residents, visitors, and first responders."
About FDNY
The Fire Department City of New York (FDNY) is the largest combined Fire and EMS provider in the United States. With more than 11,000 firefighters and 3,000 EMTs and paramedics, FDNY provides the fire protection and inspection, emergency medical, technical rescue, and biological, chemical and radioactive hazard first response services for more than 8 million citizens in an area of more than 320 square miles. Annually, FDNY responds to over 990,000 fire calls, more than 1.3 million EMS calls.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Rad-57 will provide sufficient sensitivity and specificity to detect elevated levels of carboxyhemoglobin and potentially life-threatening carbon monoxide poisoning within seconds for all patients and will provide an effective early warning system to enable timely rescue, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Radical-7® and Rad-57® Pulse CO-Oximeters™ Awarded Airworthiness Release Certification by the United States Army
Airworthiness Designation Green Lights Masimo Pulse CO-Oximeters for Military Flight Operations
Irvine, California – September 3, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced that its Radical-7 and Rad-57 Pulse CO-Oximeters have achieved Airworthiness Release (AWR) Certification from the United States Army. AWR certification is a fundamental milestone that allows the U.S. military to use Masimo's multi-function, advanced noninvasive patient monitoring devices during flight operations aboard rotary aircraft.
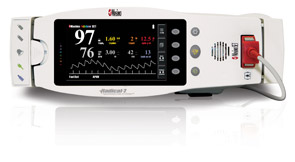 |
 |
| Masimo Radical-7® Pulse CO-Oximeter™ | Masimo Rad-57® Pulse CO-Oximeter™ |
The U.S. Army Aeromedical Research Laboratory (USAARL) completed extensive aeromedical testing and evaluation on the Radical-7 and Rad-57—including electromagnetic interference and compatibility, environmental, vibration/motion, and human factors testing—to certify that both devices meet stringent requirements to operate onboard U.S. military aircraft in flight. Meeting the Army's airworthiness testing requirements and standards affirms the durability, reliability and superior performance of both the Radical-7 and Rad-57, which are designed to excel in a wide range of demanding environments, including those faced by emergency medical services, fire, law enforcement, military field medical operations and medevac units.
Joe E. Kiani, Founder and CEO of Masimo, stated, "We are proud of our long-standing presence in military hospitals. Now, with the Radical-7 and Rad-57 both receiving airworthiness certification, U.S. troops can benefit from definitive, lifesaving physiological monitoring from the moment they need it on the ground and during transport to a medical center. Masimo is committed to supporting the military through the entire care continuum, from the battlefield to the bedside."
The Masimo Radical-7 Pulse CO-Oximeter features first-ever noninvasive and continuous hemoglobin (SpHb™) monitoring technology, which may allow combat medics to quickly and easily detect and assess the severity of internal bleeding and provide the most appropriate frontline trauma care on the battlefield. During surgery, the Radical-7 may help guide more effective transfusions and enable better blood management strategies. Radical-7 also noninvasively and continuously measures oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI™ for fluid responsiveness, oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate to quickly detect and treat potentially life-threatening conditions earlier. The Masimo Rad-57 portable, handheld Pulse CO-Oximeter accurately and noninvasively measures carboxyhemoglobin (SpCO) and methemoglobin (SpMet) levels in the blood in seconds, along with SpO2 and pulse rate to facilitate rapid, reliable treatment decisions in military settings where carbon monoxide poisoning or methemoglobinemia may be encountered.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding repeatability of the performance and results for the Radical-7 and Rad-57 Pulse CO-Oximeters during laboratory and flight operations, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Children's Hospital Oakland Standardizes to Masimo Rainbow SET® Pulse CO-Oximetry™ Technology
Pioneering Noninvasive and Continuous Hemoglobin Hospital-wide, Children's Hospital Oakland Leverages State-of-the-Art Masimo Rainbow Platform to Advance Pediatric Care
Irvine, California – September 1, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that Children's Hospital & Research Center Oakland has completed its system-wide conversions to Masimo Rainbow SET Pulse CO-Oximetry technology. Standardizing to Masimo Rainbow SET makes Children's Hospital Oakland the first children's hospital to pioneer new continuous noninvasive blood monitoring capabilities hospital-wide—allowing earlier and better clinical decisions without the intermittent pain and wait associated with drawing blood.
By converting to Masimo Rainbow SET, Children's Hospital Oakland clinicians will gain all the benefits and Measure-Through Motion capabilities of Masimo SET pulse oximetry, along with the breakthrough innovation of the first-ever continuous and noninvasive hemoglobin monitoring (SpHb™) technology. Noninvasive hemoglobin will allow clinicians at Children's to detect and treat chronic or acute anemia earlier, identify internal bleeding sooner, and more effectively manage blood transfusions for their patients. The ability to immediately detect and treat potentially life-threatening conditions earlier may help save lives and improve patient outcomes.
Katie Sabato, MS, RRT, director of Respiratory Care, Pulmonary Function & Sleep Labs at Children's Hospital Oakland, stated, "All of our efforts are focused on helping kids get better, which requires a very special level of care and some of the world's most sophisticated technology. Masimo Rainbow SET will allow us to measure critical components of a child's blood quickly and easily, without the pain of a needle stick and the discomfort of having blood drawn. It's also a great example of how we leverage advanced technology and innovative care solutions to improve comfort and care and speed-up a child's recovery."
Masimo Founder and CEO, Joe E. Kiani, stated, "We are proud of our relationship with Children's Hospital Oakland. Children's was one of the first children's hospitals to convert to Masimo SET years ago and, with a history of leadership in the practice of neonatal and pediatric care, they are continuing to lead new efforts to transform pediatric patient care with their conversion to Masimo Rainbow SET Pulse CO-Oximetry. We are delighted that they have chosen to significantly impact the care and treatment of children and advance the practice of medicine using our noninvasive and continuous Rainbow platform."
Masimo Rainbow SpHb is transforming the way patients are managed in the operating room, intensive care unit and emergency department, where clinicians are reporting earlier identification of necessary blood transfusions, reduction in unnecessary transfusions and invasive tests, increased patient safety, and improved clinical outcomes.
Amit Gupta, M.D., pediatric anesthesiologist at Children's Hospital & Research Center Oakland, stated, "Preserving safe hemoglobin levels are always a particular concern for pediatric patients who already have very little hemoglobin to spare. With Masimo Rainbow SET in the peri-operative setting we can noninvasively detect internal bleeding, fluid responsiveness, oxygen saturation, pulse rate, and perfusion—all in a matter of seconds. Having this level of physiological detail immediately available at our fingertips is a tremendous benefit that helps to speed patient assessment, treatment, care, and the safe return to optimal health."
Children's Hospital & Research Center Oakland is a regional and global resource for advanced pediatric care, research and medical education. With more than 200,000 patient visits per year, Children's has Northern California's most active pediatric trauma center, the region's busiest pediatric intensive care unit, and one of the largest sickle cell treatment and research centers in the world.
About Children's Hospital & Research Center Oakland
Children's Hospital & Research Center Oakland is Northern California's only freestanding and independent children's hospital. Children's is a leader in many pediatric specialties including neonatology, cardiology, neurosurgery and intensive care. The hospital is a designated Level 1 pediatric trauma center and has the largest pediatric critical care facility in the region. Children's Hospital has 190 licensed beds, 201 hospital-based physicians in 30 specialties, more than 2,600 employees and an annual operating budget of $312 million. Children's research arm, Children's Hospital Oakland Research Institute (CHORI), is internationally renowned for taking state-of-the-art basic and clinical research to the bedside with interventions for treating and preventing human disease. CHORI has 300 staffers, a budget of about $50 million, and is ranked among the nation's top 10 research centers in National Institutes of Health funding to children's hospitals. CHORI is a leader in translational research, developing new vaccines for infectious diseases, and discovering new treatment protocols for previously fatal or debilitating conditions including cancer, sickle cell anemia, thalassemia, diabetes, asthma, HIV/AIDS, pediatric obesity, nutritional deficiencies, birth defects, hemophilia and cystic fibrosis. For more information, go to www.childrenshospitaloakland.org.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo Rainbow SET will provide sufficient sensitivity and specificity to detect physiological abnormalities in real-time, enabling clinicians to detect and identify potentially life-threatening conditions earlier and make better clinical and treatment decisions for their patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at the Thomas Weisel Partners Healthcare Conference 2009
IRVINE, Calif., August 31, 2009 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced that its management is scheduled to present at the Thomas Weisel Partners Healthcare Conference 2009 at the Four Seasons Hotel in Boston, Massachusetts, on Thursday, September 10, 2009, at 10:20 a.m. EDT. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET® provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at http://www.masimo.com.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Childrens Hospital Los Angeles Completes Conversion to Masimo SET® Pulse Oximetry Technology
Irvine, California – August 25, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that Childrens Hospital Los Angeles, ranked by U.S. News & World Report as one of 'America's Best Children's Hospitals', has completed its system-wide conversion to Masimo SET pulse oximetry technology.
Masimo SET has been clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 and pulse rate measurements, even under the most difficult clinical conditions of patient motion and low peripheral perfusion—conditions common to infants and children. Its unmatched sensitivity and specificity, combined with a validated accuracy to oxygen saturations as low as 60% on patients, including cyanotic patients, helps clinicians keep their tiniest, most vulnerable patients safe. Masimo SET pulse oximetry aids the detection of congenital heart disease (CHD) and helps significantly reduce eye damage and blindness caused by retinopathy of prematurity (ROP)—one of the most common causes of visual impairment in neonatal patients.1-5
According to Randall Wetzel, M.D., Chair of Anesthesiology and Critical Care Medicine at Childrens Hospital Los Angeles, "Standardizing to Masimo SET allows us to more accurately assess oxygenation in children to detect life-threatening conditions earlier. It also provides a unique platform for growth with the opportunity to use Masimo Rainbow SET noninvasive measurements, such as continuous hemoglobin measurement. The ability to measure hemoglobin continuously during surgery will enable us to identify trends earlier and to more quickly and effectively manage blood and fluid administration interoperatively and in the ICUs."
Masimo Founder and CEO, Joe E. Kiani, stated, "We are happy that Childrens Hospital Los Angeles has selected Masimo SET as their pulse oximetry technology and Pulse CO-Oximetry platform. Given what our technology has been proven to do for patients, it's always gratifying to convert a hospital to Masimo, but it is especially gratifying when it is one of the world's best children's hospitals right here in Southern California."
Childrens Hospital Los Angeles, a Level I Pediatric Trauma Center and Level III Neonatal Intensive Care Unit, treats over 93,000 children each year who need care that is so complex, it can only be provided by medical experts. With one of the largest dedicated neonatal/pediatric transport programs in the nation and the only dedicated Cardiothoracic Intensive Care Unit on the west coast, Childrens Hospital provides innovative therapies for high-risk infants transferred from other hospitals throughout Southern California and beyond.
In the past five years, approximately 900 hospitals in the U.S. have converted completely to Masimo. In addition, more than half of the top hospitals listed on the U.S. News & World Report 'Best Hospitals Honor Roll'—including four of the top five—rely on Masimo SET pulse oximetry to provide the best care to their patients.
1 de Wahl Granelli A, et al. "Screening for Duct-Dependent Congenital Heart Disease with Pulse Oximetry: A Critical Evaluation of Strategies to Maximize Sensitivity." Acta Paediatrica 2005; 94: 1590-1596.
2 Granelli AW, et al. "Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction." Acta Paediatrica 2007; 96: 1455-1459.
3 Anne de-Wahl Granelli, et al., "Impact of Pulse Oximetry Screening on the Detection of Duct Dependent Congenital Heart Disease: a Swedish Prospective Screening Study in 39,821 newborns." BMJ. 2009; 338;a3037.
4 Armando R. Castillo, Richard Deulofeut, Augosto Sola. "Clinical Practice and SpO2 Technology in the Prevention of ROP in ELBW Infants." Publication 8440.7; 2007 Pediatric Academic Societies.
5 Tsutum T, et al. "Clinical Evaluation of Accuracy of Masimo LNOP Blue Sensor in Cyanotic Infants." Critical Care Medicine 2006; 34(12): A56.
About Childrens Hospital Los Angeles
Founded in 1901, Childrens Hospital Los Angeles has been treating the most seriously ill and injured children in Los Angeles for more than a century, and it is acknowledged throughout the United States and around the world for its leadership in pediatric and adolescent health. Childrens Hospital is one of America's premier teaching hospitals, affiliated with the Keck School of Medicine of the University of Southern California since 1932. The Saban Research Institute of Childrens Hospital Los Angeles is among the largest and most productive pediatric research facilities in the United States. Since 1990, U.S. News & World Report and its panel of board-certified pediatricians have named Childrens Hospital Los Angeles one of the top pediatric facilities in the nation. Childrens Hospital Los Angeles is one of only 10 children's hospitals in the nation – and the only children's hospital on the West Coast – ranked in all 10 pediatric specialties in the U.S. News & World Report rankings and named to the magazine's "Honor Roll" of children's hospitals.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of results obtained by Childrens Hospital Los Angeles, risks related to our belief that Masimo SET will deliver sufficient sensitivity and specificity to improve patient monitoring and will achieve accuracy in oxygen saturation as low as 60% on patients, including cyanotic patients, and risks related to enabling detection of CHD and ROP, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


GS GmbH Signs Technology License Agreement to Integrate Masimo Rainbow SET® Pulse CO-Oximetry™ into Defibrillators and Patient Monitoring Systems
EMS Market Leader from Germany Standardizes on Masimo's Revolutionary Rainbow SET Pulse CO-Oximetry Technology Platform
Irvine, California and Kaufering, Germany – August 20, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, and GS Elektromedizinische Geräte G. Stemple GmbH, a German developer and manufacturer of innovative medical equipment for the Emergency Medical Services (EMS), today jointly announced a worldwide technology licensing agreement to integrate Masimo Rainbow SET Pulse CO-Oximetry into corpuls® patient monitoring modules. The agreement enables GS GmbH to expand the vital signs and noninvasive measurement capabilities of its defibrillators and patient monitoring systems to help EMS professionals detect and treat life-threatening conditions earlier.
The breakthrough noninvasive blood constituent monitoring capabilities of Masimo Rainbow SET Pulse CO-Oximetry allow for continuous monitoring of hemoglobin, carbon monoxide, methemoglobin, PVI for fluid responsiveness, perfusion index, and measure-through motion pulse oximetry. With Masimo Rainbow SET, the ability to detect internal bleeding, carbon monoxide poisoning, methemoglobinemia, fluid responsiveness, perfusion, saturation, and pulse rate quickly, easily and continuously can help save lives and improve patient outcomes.
According to Klaus Stemple, General Manager of GS GmbH, the key to "developing better lifesaving support solutions and techniques is to stay ahead of the mainstream. Our corpuls products are innovative leaders in the defibrillator market and the integration of Masimo Rainbow SET Pulse CO-Oximetry measurements is another consistent step in the design and manufacture state-of-the-art defibrillator/monitoring-systems that spearhead the industry in patient monitoring performance. The combination of corpuls and Masimo Rainbow SET creates a unique defibrillator solution for EMS professionals looking to leverage additional vital parameters to more thoroughly assess a patient's physiological status and make better clinical and treatment decisions."
The corpuls3—a unique 3-in-1 modular defibrillator system combining three components including: a compact patient box, a large monitoring unit, and a defibrillator/pacer unit — will be one of the first products to feature Masimo Rainbow SET Pulse CO-Oximetry capabilities. The Masimo Rainbow SET measurement option will be available in mid-2010.
Masimo Founder and CEO, Joe E. Kiani, stated, "GS GmbH was one of the first European equipment manufacturers to license Masimo SET pulse oximetry technology over 10 years ago. Today, they are still a market leader in providing innovative EMS solutions throughout Europe and, with this technology license agreement, will further expand the availability of Masimo Rainbow SET Pulse CO-Oximetry to the benefit of patients and EMS professionals alike."
About GS GmbH
GS Elektromedizinische Geräte G. Stemple GmbH is a German company and internationally-operated business specializing in the development and manufacture of innovative high-end medical devices for emergency medical services and intensive care for over 25 years. The corpuls® defibrillators and patient monitoring systems are robust, reliable and durable devices designed for use in the first aid treatment of people suffering from sudden cardiac arrest. Additional information about corpuls and its products may be found at www.corpuls.com.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the timing and commercial availability of corpuls3 featuring Masimo Rainbow SET Pulse CO-Oximetry technology, risks related to our belief that Masimo Rainbow SET will provide sufficient sensitivity and specificity to detect physiological abnormalities in real-time, enabling EMS professionals to detect and identify potentially life-threatening conditions earlier and make better clinical and treatment decisions for their patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. Corpuls and Corpuls3 are trademarks or registered trademarks of GS Elektromedizinische Geräte G. Stemple GmbH.


Masimo and the International Association of Fire Chiefs Launch Nationwide Health & Safety Program to Reduce Firefighter Death and Injury
Program Translates New Industry Standards into Functional Procedures and Operational Processes to Combat Carbon Monoxide (CO) Health Risks and Other Duty-Related Hazards
Irvine, California – August 18, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, and the International Association of Fire Chiefs (IAFC) jointly announce a new health and safety program designed to help fire departments across the nation implement new rehabilitation and medical monitoring standards to reduce the duty-related health hazards that claim the lives of hundreds of the nation's firefighters each year. The IAFC Firefighter Rehabilitation and Medical Monitoring Program provides the tools, training and resources fire departments need to put new national standards into practice.
Numerous published studies have shown that heart damage resulting from CO poisoning is a frequent and significant predictor of mortality, requiring appropriate screening for the detection of such injuries.1-4 Last year, the National Fire Protection Association (NFPA) revised its national codes and standards for Section 1584, "Standard on the Rehabilitation Process for Members During Emergency Operations and Training Exercises," to include the routine use of CO screening.
"Evidence shows that mortality and morbidity increase following CO exposure, which is the most commonly encountered contaminant found in environmental studies of firefighters," stated Mike McEvoy, EMS Director for the New York State Association of Fire Chiefs. "However, a significant number of these injuries and deaths are preventable, simply through the implementation of careful medical monitoring and thorough rehabilitation efforts."
The new IAFC Firefighter Rehabilitation and Medical Monitoring Program, jointly developed and sponsored by Masimo and the IAFC, addresses vital occupational health and safety hazards for firefighters to meet a previously unmet need without draining department resources. The program offers free onsite and online training, educational resources and materials, development tools for standard operating procedures, guidance, and support to help fire departments implement and comply with NFPA 1584 required standards.
"This program provides fire departments with the materials they need to properly implement the national codes and standards, and train their members," said IAFC President Chief Larry J. Grorud, CFO, MIFireE. "Any delay or failure to implement these critical health and safety measures could increase injury and death rates for firefighters and reduce work capacities, manpower and efficiency for their departments."
NFPA 1584 mandates that "Any firefighter exposed to CO or presenting with headache, nausea, shortness of breath, or gastrointestinal symptoms at an incident where CO is present should be assessed for carbon monoxide poisoning."
The program identifies eight key elements for effective firefighter rehabilitation at incident scenes and training exercises, including: relief from environmental conditions, rest and recovery, temperature regulation, hydration, calorie and electrolyte replacement, medical monitoring, member accountability and tracking systems, and release for return to duty.
Masimo Founder and CEO, Joe E. Kiani, stated, "We are proud to work with the IAFC on this Firefighter Rehabilitation and Medical Monitoring Program. Our goal is to make it easier for fire departments to put the procedures and resources in place to meet the rehabilitation needs of their firefighters at every incident scene. The more successful we are, the safer firefighters will be."
1 Hampson NB, Rudd RA, Hauff NM. "Increased Long-term Mortality Among Survivors of Acute Carbon Monoxide Poisoning." Crit Care Med. June 2009; 37(6):2116-8.
2 Williams J, Lewis II RW, Kealey GP., "Carbon Monoxide Poisoning in Myocardial Ischemia in Patients with Burns." J Burn Care Rehabilitation. 1999; 12:210-213.
3 Hedblad B, Engstrom, Janzon E, Berglunf G, Janzon L. "COHb% as a marker of cardiovascular risk in never smokers: Results from a population-based cohort study." Scand J Pub Health. 2006;34:609-615.
4 Dueas-Laita A., Perz-Castrilln J. L., Ruiz-Mambrilla M., Raymond L. W., Barringer T. A., Konen J. C., Kales S. N., Soteriades E. S., Christiani D. C. "Heart Disease Deaths among Firefighters." N Engl J Med 2007; 356:2535-2537, Jun 14, 2007.
About the International Association of Fire Chiefs (IAFC)
IAFC represents the leadership of over 1.2 million firefighters and emergency responders. IAFC members are the world's leading experts in firefighting, emergency medical services, terrorism response, hazardous materials spills, natural disasters, search and rescue, and public safety legislation. Since 1873, the IAFC has provided a forum for its members to exchange ideas and uncover the latest products and services available to first responders. www.iafc.org
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumption that Pulse CO-Oximetry will become the preferred method of noninvasive CO assessment for the IAFC and NFPA, risks related to our belief that SpCO will improve the clinical assessment of CO poisoning in firefighters and offer a measureable improvement over existing medical monitoring capabilities to enhance firefighter rehabilitation efforts, as well as other factors discussed in the "Risk Factors" section of our most recent reports, filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks,
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo and Oridion Sign Connectivity Agreement to Integrate Capnostream™20 Portable Bedside Monitors into the Masimo Patient SafetyNet™ System
Joint Development of a Two-way Interface Enabling Wired and Wireless Communication Now Underway
Irvine, California/Jerusalem, Israel – August 11, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, and Oridion (SIX Swiss Exchange: ORIDN), a global innovator of capnography monitoring solutions, today jointly announced an agreement to establish compatibility and connectivity between Oridion Capnostream™20 portable bedside monitors and the Masimo Patient SafetyNet™ remote monitoring and clinician notification system. The agreement provides for joint development of a two-way interface allowing Patient SafetyNet to retrieve, analyze, and display physiological data from Capnostream monitors, while managing bi-directional communication and alarm features to facilitate earlier recognition of and clinical response to adverse respiratory events that require immediate clinical intervention.
According to a recently released HealthGrades study on patient safety in American hospitals, failure to rescue—which is defined as death occurring when an adverse clinical event is not recognized in time to save a patient with serious treatable complications—is the most common and most serious medical error at U.S. hospitals. Masimo Patient SafetyNet was specifically developed to provide a new level of safety to patients on general care floors, where nurse-to-patient ratios can often preclude the level of direct surveillance required and recommended to preempt adverse events.
The key to a successful patient rescue is getting the right information to the right clinician at the right time. This means that accurate, reliable, and actionable patient data is necessary for rapid response or patient safety solutions to be effective. Oridion Capnostream monitors feature two industry-leading technologies—Oridion's patented Microstream® sidestream Smart Capnography™, and Masimo SET® Measure-Through Motion and Low-Perfusion pulse oximetry—that together provide clinicians with an early warning of a possible life-threatening deterioration in their patients' physiological status.
Oridion Microstream technology enables the most accurate and reliable assessment of breathing quality for patients requiring continuous ventilation monitoring, providing clinicians with timely recognition of changes that may lead to a decline in respiratory status or airway compromise. Masimo SET is the most accurate and reliable pulse oximetry technology, clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. Masimo Patient SafetyNet will be the first remote monitoring and clinician notification system to feature Oridion's newest Smart Capnography™ algorithm—Integrated Pulmonary Index™ (IPI)—enabling real-time tracking and trending of EtCO2, respiration rate, pulse rate, and SpO2 for an inclusive assessment of the patient's ventilatory status with a single index parameter.
According to Gerry Feldman, President of Oridion Systems Ltd., a partnership between gold standard capnography and Masimo pulse oximetry "represents a winning combination for patients, clinicians and hospitals alike because a change in breathing quality and oxygenation can be important early indications that a patient may be heading for big trouble. The ability to quickly route this vital information to a qualified caregiver or rapid response team for appropriate clinical intervention can mean the difference between a timely rescue and failure to rescue. Oridion Microstream and Masimo SET technologies, together with the Masimo Patient SafetyNet system, will arm hospitals with the accurate, real-time information they need to make the most of their patient safety efforts."
Masimo Founder and CEO, Joe E. Kiani, stated, "We are happy to partner with Oridion to expand the variety of vital sign parameters and third-party multi-parameter patient monitoring devices that offer real-time wireless connectivity to the Masimo Patient SafteyNet remote monitoring and clinician notification system. The Oridion Capnostream 20 monitor combines proven best-in-class measurements for oxygenation and ventilation in a portable and easy-to-use device for clinicians and patients in both hospital and outpatient settings. Enabling full connectivity and two-way wireless communication between Oridion Capnostream monitors and Masimo's Patient SafetyNet system will provide additional flexibility and monitoring options for healthcare facilities looking to advance patient safety and improve clinical outcomes."
Development of the two-way interface enabling full connectivity between Oridion monitors and Patient SafetyNet is now underway, with completion expected in Q4 of this year. Furthermore, existing Oridion Capnostream 20 monitors and Masimo Patient SafteyNet systems can be easily upgraded via software to add this functionality following full commercial release of the bi-directional interface.
About Oridion
Oridion Systems Ltd. (www.oridion.com) is a global medical device company specializing in patient safety monitoring. The Company operates through wholly owned subsidiaries in the United States, Europe, and Israel. Oridion develops proprietary medical devices and patient interfaces, based on its patented Microstream® technologies, for the enhancement of patient safety through the monitoring of the carbon dioxide (CO2) in a patient's breath. These products provide effective, proven airway management and are used in various clinical environments, including procedural sedation, pain management, operating rooms, critical care units, post-anesthesia care units, emergency medical services, transport, alternate care and other settings where patients' ventilation may be compromised and at risk. Additional information about Oridion and its products may be found at www.oridion.com.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the timing and availability of connectivity and a two-way interface between Oridion Capnostream and Masimo Patient SafetyNet, risks related to our belief that Patient SafetyNet will provide an effective early warning system to enable timely rescue, risks related to our belief that Masimo SET will provide sufficient sensitivity and specificity to detect physiological abnormalities and potentially life-threatening conditions in real-time for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
+1 (949) 297-7348
Walter Tabachnik
Oridion Systems Ltd.
+972 (2) 589-9159
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. Capnostream and Microstream are trademarks or registered trademarks of Oridion.


Masimo Reports Second Quarter 2009 Financial Results
Q2 2009 Highlights:
- Earnings per share increased by 22% to $0.22 per share from $0.18 in prior year
- Product revenues increased 13% to $70.0 million from $62.1 million in prior year
- Rainbow revenues increased 56% to $4.5 million from $2.9 million in prior year
Irvine, California, August 4, 2009 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced its financial results for the second quarter of 2009.
For the second quarter of 2009, Masimo reported product revenues of $70.0 million representing a 13% increase over $62.1 million for the second quarter of 2008. Including royalty revenues, Masimo reported total 2009 second quarter revenues of $83.6 million compared to $74.8 million for the second quarter of 2008. For the second quarter of 2009, Masimo reported earnings per share of $0.22 compared to $0.18 per share for the second quarter of 2008.
Masimo reported that it shipped 27,100 Masimo SET and Masimo Rainbow SET oximetry units, excluding handheld units, during the second quarter of 2009, resulting in an estimated 605,000 of Masimo SET and Masimo Rainbow SET pulse oximeters in use worldwide. In the second quarter of 2009, revenues from Masimo Rainbow SET products increased to $4.5 million from $2.9 million in the same prior year quarter.
For the first six months of 2009, Masimo reported product revenues of $144.5 million representing a 19% increase over $121.8 million for the first six months of 2008. Including royalty revenues, Masimo reported total 2009 year to date revenues of $169.1 million, representing a 16% increase compared to $145.9 million for the prior year period. For the first half of 2009, Masimo reported earnings per share of $0.43, up 34% from $0.32 per share for the first half of 2008.
Joe E. Kiani, Chairman and Chief Executive Officer of Masimo, said, "We had a good quarter given the recession—while revenues were down quarter over quarter, they grew 13% year over year, thanks to continued strong Masimo SET growth and even stronger Masimo Rainbow SET growth. These results underline both the resilience of our business model and the power of our innovation in noninvasive monitoring, which is helping our customers improve and automate patient care."
Masimo also reported that as of July 4, 2009, cash and cash equivalents totaled $156.6 million, up from $146.9 million at January 3, 2009.
Financial Guidance
While providing financial guidance in this economic environment continues to be more difficult than in prior periods, Masimo is reaffirming its 2009 earnings guidance of $0.83 to $0.87 per share; however, Masimo is adjusting its total 2009 product revenue guidance to between $295 million and $300 million, down from its prior guidance of $310 million to $315 million. Masimo also expects its 2009 royalty revenues to be in the range of $44 million to $46 million, compared to prior guidance of $42 million to $46 million. For the full year 2009, Masimo now expects its total revenues to be between $339 million and $346 million, down from $352 million to $361 million. The projections and guidance set forth above are estimates only and actual results could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation number for both dial-in numbers is 21245431. A live web cast of the conference call will be available online from the "Investor Relations" page of the Company's corporate web site at www.masimo.com. After the live web cast, the call will remain available on Masimo's website through September 4, 2009. In addition, a telephonic replay of the call will be available until August 18, 2009. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 21245431.
About Masimo
Masimo (NASDAQ: MASI) develops, manufactures and markets innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the Company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical and laboratory studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®) and plethysmograph variability index (PVI®), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com. Any information contained in, or that can be accessed through, our website is not incorporated by reference into, nor is it in any way a part of, this release.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations, prospects and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and estimates for total revenues, including royalty and product revenues, and earnings per share for the full fiscal year 2009. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo Rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any new products and technologies of ours; obtaining regulatory approval of our current and future products and technologies, including the recently announced total hemoglobin measurement; the risk that the implementation of our international realignment, even if timely implemented, will not produce the anticipated operational and financial benefits, including a lower effective tax rate; the loss of our customers the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website, at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
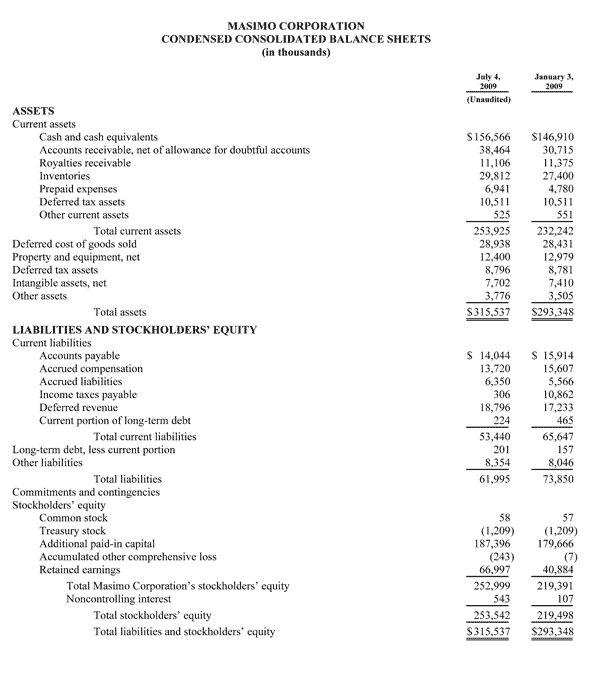
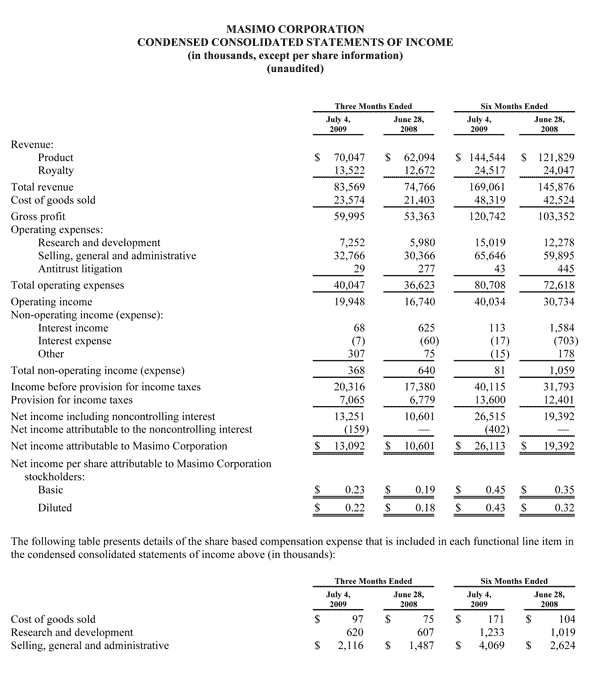
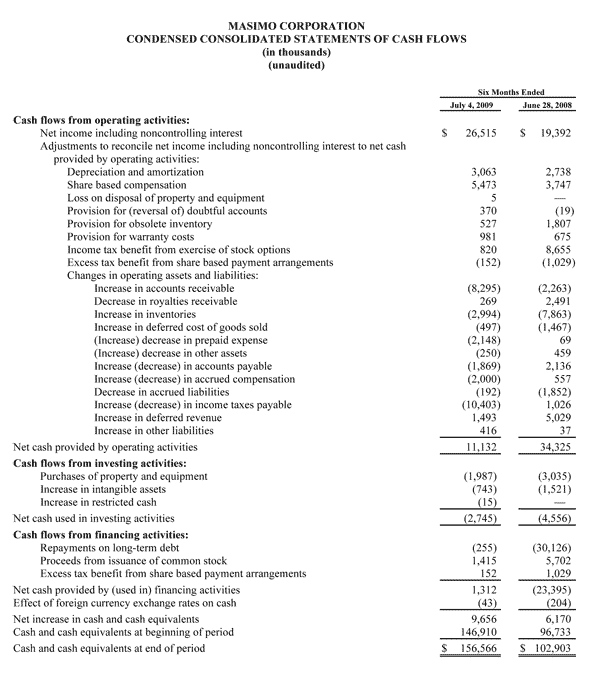


The International Association of Fire Fighters and Masimo Team Up on a New Global Campaign to Raise Awareness of Carbon Monoxide-Related Death and Injury Risks for Firefighters
New Carbon Monoxide (CO) Safety Campaign Featuring Actor Randolph Mantooth from the Hit TV Show EMERGENCY! Urges Education, Protection and Safety
Irvine, California – July 23, 2009 – The International Association of Fire Fighters (IAFF) and Masimo jointly announced the launch of a new international health and safety initiative aimed at educating firefighters about the duty-related dangers of carbon monoxide (CO) and reducing known risk factors that unnecessarily kill and injure hundreds of firefighters each year. The campaign, kicked off at the biennial IAFF EMS Conference in Miami Beach, Florida, will feature actor Randolph Mantooth — widely recognized as Los Angeles County firefighter/paramedic "Johnny Gage" in the popular 1970s television series EMERGENCY! — speaking out about his own near-death CO experience while highlighting the immediate and long-term health risks and prevention strategies of CO exposure at IAFF district meetings and conferences throughout the year.
CO poisoning is a danger at every fire, but its symptoms — headache, dizziness, fatigue — are often absent or non-specific, making on-scene awareness and detection difficult. This puts firefighters at significant risk on the scene of a fire because even mild CO poisoning can rob the brain of oxygen,1 which can lead to poor decision making.2 It can also rob the heart of oxygen, causing immediate life-threatening complications — with half of on-duty firefighter deaths being attributed to heart attacks or stroke.3 And, just one severe CO poisoning almost doubles their long-term risk of death.4 CO poisoning is often present without symptoms and is not easy to detect,5 — making firefighter education and awareness a critical necessity. The new educational campaign builds on last year's efforts to increase CO awareness and safety among firefighters by distributing education materials to more than 3,200 local union presidents in the United States and Canada.
"We are pleased to team with Masimo and with Randolph Mantooth in this important effort," said IAFF General President Harold Schaitberger. "This is a critical educational endeavor that we hope will help contribute to the protection of our members."
Mantooth's involvement is personal: "Since my own near-death CO experience and subsequent rescue by two firefighter/paramedics, I've become an advocate of firefighters, paramedics, EMTs, and other emergency responders as a first step toward repaying my debt to these brave men and women. I want to encourage them to protect their own lives, so they can be there to protect others."
Randy's new IAFF presentation encourages firefighters to get their CO levels tested on the fire scene and, if elevated, seek prompt treatment. He also urges firefighters to take personal responsibility for their safety by recognizing the dangers of CO and preventing unnecessary exposures.
"The trade-offs for ignoring the serious dangers of CO exposure are heart attack, stroke, loss of motor skills, lifelong disability, or death," Mantooth explained. "Our firefighters need to know and understand these significant occupational hazards, how to properly protect themselves and prevent unnecessary health risks to improve the odds that they will be around tomorrow to do what they were born to do."
As part of the campaign, Randy has created a new web site — www.COsafetynet.org — to provide immediate and free access to valuable information and educational resources designed to increase awareness of the immediate personal safety, long-term health, and departmental risks associated with CO exposure for firefighters. The web site will provide fast facts and other important information about specific occupational exposures to CO and associated risks to firefighter health and safety, as well as links to resources designed to help keep firefighters safe from the dangers of CO poisoning.
Joe E. Kiani, Chairman and CEO of Masimo, stated, "Fire fighters, within their normal course of work, are routinely exposed to dangerous levels of CO and bear the largest risks to their own health and safety. We are happy to partner with the IAFF and Mr. Mantooth to introduce this special CO education and outreach campaign to save the lives and preserve the health of all firefighters from the potentially life-threatening effects of CO."
1 Bledsoe, BE: "The Perils of CO" FireRescue Magazine. September 2005.
2 Jakubowski, G. The Invisible Incidents: How to respond to CO alarms. FireRescue Magazine. 22(11):52–55, 2004.
3 Bledsoe, BE. "The Dangers of CO: Understanding Cardiovascular Risks to Responders from CO Exposure." Journal of Emergency Medical Service. 32:54-59, 2007.
4 Hampson, NB et al. "Increased long term mortality among survivors of acute carbon monoxide poisoning." Crit Care Med. 2009; 37(6): 1941-47.
5 Hampson, NB, et al: "Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture?" American Journal of Emergency Medicine. 26:665-669, 2008.
About IAFF
The International Association of Fire Fighters is a member driven union — for firefighters, by firefighters — representing more than 295,000 full-time professional firefighters and paramedics who protect 85 percent of the nation's population. With one of the most active lobbying organizations in Washington, IAFF serves as the primary advocate for providing firefighters and paramedics with the tools they need to perform their jobs and is the driving force behind nearly every advance within the fire and emergency services for the past 90 years. With more than 3,200 affiliates protecting communities in every state of the United States and in Canada, the IAFF has established professional standards for the North American fire service and provides a strong voice in the development and implementation of new training programs and equipment. The IAFF in no way endorses, sponsors, or is otherwise involved in the design, manufacture, sale or distribution of goods and services by the Masimo Corporation. No representation, warranty or condition, express or implied, statutory or otherwise is given or assumed by the IAFF in respect to goods and services designed, manufactured, sold and/or distributed by the Masimo corporation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the superior sensitivity and specificity of Masimo Rainbow SET Pulse CO-Oximetry technology will improve the detection of elevated CO blood levels over alternative methods and facilitate rapid detection of CO poisoning during firefighter rehabilitation, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended April 4, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks,
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report Second Quarter 2009 Financial Results on August 4, 2009
Conference call and webcast to begin after markets close at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., July 22, 2009—Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced it will release second quarter 2009 financial results for the period ended July 4, 2009 after the market closes on Tuesday, August 4, 2009.
A conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe E. Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the Company's corporate web site at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation number for both dial-in numbers is 21245431. After the live webcast, the call will remain available on Masimo's website through September 4, 2009. In addition, a telephonic replay of the call will be available through August 18, 2009. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 21245431.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at http://www.masimo.com.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Receives FDA Clearance for New Rainbow® ReSposable™ Sensor System
Innovative Combination Reusable-Disposable Sensor System Provides Cost-Effective & Environmentally Green Pulse CO-Oximetry Monitoring
Irvine, California – July 21, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced the FDA 510(k) clearance of its new Masimo Rainbow ReSposable™ Sensor System, a revolutionary new sensor system that offers the performance and comfort of a single-use adhesive disposable sensor with the cost-effectiveness and environmentally-green advantages of a reusable sensor. The new sensor system provides continuous and noninvasive measurements of hemoglobin (SpHb™), oxygen content (SpOC™), PVI™ for fluid responsiveness, and methemoglobin (SpMet®), in addition to oxygen saturation (SpO2), perfusion index, and pulse rate—at savings of about 50% compared to the existing single patient use adhesive Rainbow sensor.
The Rainbow ReSposable Sensor System combines the best features of Masimo LNOP®, LNCS®, and Rainbow® sensors into an innovative two-piece design that includes a reusable sensor—enabling the portion that connects to the patient cable to be used on multiple patients - while the adhesive disposable sensor that attaches directly to the patient's finger is used on only one patient. This dramatically minimizes waste, enabling hospitals to reduce their costs safely, and effectively fulfill their 'green' initiatives.
The reusable portion of the two-piece system provides multi-patient use for cost-effectiveness, while the flexible cable design maximizes patient comfort, and enables easy, one-step cleaning. The snap-in-place connector design allows easy connection and reconnection to the same patient—and reuse on the next patient. The single-use disposable portion of system features an adhesive that keeps emitter and detector positions stable against the skin to assure the best in Masimo SET and Rainbow SET measurement performance. The design maximizes fit and comfort for the patient, while the protective covering minimizes contact with patient to reduce cross contamination risk.
"We have tried the Masimo Rainbow ReSposable System and found that these sensors provided us with all of the benefits and superior performance of Masimo's continuous and noninvasive Rainbow measurements, including noninvasive hemoglobin monitoring, along with the cleanliness and patient comforts of the standard single patient use sensor at a fraction of the cost and waste," stated Bertrand Debaene, M.D., Department of Anesthesia at the University Hospital Center in Poitiers, France.
"The Masimo Rainbow ReSposable Sensor System is based on more than ten years of research and development stemming from the feedback of hundreds of clinicians who told us what they wanted most in a sensor—less waste and more value with superior performance," stated Masimo Founder and CEO, Joe E. Kiani. "We are happy that by combining the performance and patient comfort of a single-use adhesive sensor with the cost effectiveness of a reusable sensor, the Masimo Rainbow ReSposable Sensor System also minimizes medical waste and its impact on the environment."
A Capgemini study released earlier this year showed that a typical 500-bed hospital incorporating Masimo Rainbow SET Pulse CO-Oximetry into its clinical standards and care pathways could generate nearly $500,000 in net annual cost savings and financial gains. With the Masimo Rainbow ReSposable Sensor System, the potential savings to hospitals could increase to nearly $700,000 annually.

About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the features, function and design of the Masimo Rainbow ReSposable Sensor System will enable hospitals to reduce associated waste and costs, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Announces Settlement of Lawsuit
Irvine, California – July 17, 2009 – Masimo Corporation (NASDAQ: MASI), today announced that it has reached a settlement with Respironics, Inc. of pending litigation under which Masimo and Respironics will dismiss all claims and counterclaims currently pending in the litigation between them in California Superior Court. As part of the settlement, Respironics and Masimo have agreed upon a schedule for the phase-out of the MARS oximetry technology.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors. Important factors that may cause such a difference for Masimo in connection with our settled litigation with Respironics, include, but are not limited to realizing the expected increased placement of Masimo pulse oximeters as a result of the settlement, and the expected expansion of our business relationship with Respironics; the impact of our lawsuit against Philips, given that Philips owns Respironics; and, the risks associated with the litigation being dismissed without prejudice. Additional factors that may cause Masimo's actual results to differ materially and adversely from those expressed in forward-looking statements include, but are not limited to: those factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended April 4, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Mark P. de Raad
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Rad-87® Pulse CO-Oximeter™ Garners Silver 2009 Medical Design Excellence Award
Award Honors Product Innovation, Design and Engineering Achievement, End-user Benefit, and Cost-effectiveness in Manufacturing and Healthcare Delivery
Irvine, California – July 14, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, has received the 2009 Silver Medical Design Excellence Award in the general hospital devices and therapeutic products category for the Masimo Rad-87 Pulse CO-Oximeter. The award recognizes Rad-87's excellence in medical technology engineering and design as an intuitive bedside patient monitor that helps improve clinical efficiency and keep patients safe when a clinician can't be at the bedside.
 |
The Masimo Rad-87 features the breakthrough noninvasive blood constituent monitoring capabilities of Masimo Rainbow SET® Pulse CO-Oximetry for continuous monitoring of hemoglobin, carbon monoxide, methemoglobin, PVI (fluid responsiveness), perfusion index, and measure through motion pulse oximetry with upgradability to acoustic respiration rate monitoring. Its built-in 802.11a/b/g radio allows wireless communication with the Masimo Patient SafetyNet™ remote monitoring and clinician notification system to provide patients with an enhanced level of safety on general care floors. Designed for ease-of-use, the Rad-87 allows the features clinicians need most often to be accessed with a single touch. And, as the first Masimo Rainbow SET device with a bright white LED-based display, its high contrast visual display allows measurements to be clearly seen from across the room, while an innovative new device profile feature lets clinical settings be customized and color-coded for a particular care area.
Masimo Founder and CEO, Joe E. Kiani, stated, "It is an honor to receive our third Medical Design Excellence Award for product design and engineering innovations that improve healthcare delivery and advance medical practices. We received the Gold Medical Design Excellence Award in 2006 for the Masimo Rad-57 Pulse CO-Oximeter and in 2001 for the Masimo Radical Signal Extraction pulse oximeter."
The Medical Design Excellence Awards (MDEA) competition is the only awards program that exclusively recognizes advances in the design of medical products and the achievements of medical device manufacturers responsible for the groundbreaking innovations that are changing the face of healthcare. 2009 MDEA winners were announced and honored at the June 10th ceremony during the Medical Design & Manufacturing (MD&M) East Conference and Exposition in New York. For more information about the Medical Design Excellence Awards—including details about the winning products—visit www.MDEAwards.com.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the features, function and design of the Masimo Rad-87 Pulse CO-Oximeter will enable clinicians to detect and treat life-threatening conditions earlier, improve patient outcomes, increase clinical efficiency gains, and reduce the cost of care for patients, risks related to our belief that Masimo Rainbow SET Pulse CO-Oximetry technology will provide a sufficient level of sensitivity and specificity to facilitate the noninvasive, continuous measurement of multiple blood constituents, fluid assessment and physiological parameters, risks related to our assumption that the combination of Masimo Rad-87 and Masimo Patient SafetyNet will help clinicians keep patients safe on general care floors, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Studies Presented at European Society of Anaesthesia Further Validate Masimo PVI™ for Noninvasive and Continuous Fluid Monitoring
Irvine, California – June 12, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that three new independent studies demonstrating the clinical accuracy and utility of Masimo PVI as a noninvasive and continuous measure of patient fluid status and responsiveness were presented this week at the European Society of Anaesthesiology (ESA) Annual Congress in Milan, Italy.
Although fluid administration is critical to optimizing patient status and enabling end organ preservation, unnecessary fluid administration is associated with increased morbidity and mortality. Traditional invasive measurements such as central venous pressure are not reliable to predict whether a patient will benefit from fluid administration, and newer more reliable methods to predict fluid responsiveness are also invasive and costly. Multiple recent studies have shown that Masimo PVI provides a simple and cost-effective method for accurate, noninvasive, and continuous monitoring of fluid responsiveness. The three studies presented at the ESA reinforce the accuracy of PVI compared to invasive measures and highlight its value for before, during, and after anesthesia.
Does the Pleth Variability Index correlate with stroke volume variation?
Researchers in the Department of Anesthesiology at the Hamamatsu University School of Medicine in Hamamatsu-City, Japan, compared the accuracy of Masimo PVI measurements with Stroke Volume Variation (SVV) obtained via an invasive catheter (Flo Trac™) in 13 patients. After analyzing data recorded at five points—in the supine position, lateral position, start and end of one-lung ventilation, and in the supine position again—researchers found a significant correlation between PVI and SVV (r = 0.75; p = 0.02). The study concluded that "Our results suggest that an accurate prediction of fluid responsiveness can be obtained non-invasively using the PVI." 1
The Change of Upper Limbs PVI in Spinal Block (Comparison in High Spinal Block and Non-High Spinal Block)
At the Tokai University School of Medicine in Tokyo, Japan, researchers examined how PVI in the upper limbs of patients undergoing caesarean procedures for hernia surgery changed in response to the level of spinal block reached. PVI was recorded in all patients before and every two minutes after spinal block was performed. In patients reaching the anesthetic level C-area—identified as the high spinal block group—PVI decreased. In patients who did not reach the C-area—identified as the non-high spinal block group—PVI of the upper limbs did not change significantly. Researchers summarized that high spinal block dilates vessels and increases blood flow in the upper limbs, which in turn, is shown by decreased PVI.2
Perfusion Index and Pleth Variability Index After Administration of General Anesthetic Agents
In this study, anesthesiologists at the Teikyo University School of Medicine in Tokyo, Japan, analyzed changes in PVI and Masimo perfusion index (PI) in 21 surgical patients before and after administration of general anesthetic agents. After administration of general anesthetic agents and 10ml/kg/hr of fluid during induction, PVI significantly decreased from 22.9±8.1 to 17.1±7.2 (p<0.05) and PI significantly increased from 2.1±1.7 to 3.8±2.3 (p<0.001), leading researchers to conclude that "an increase in peripheral perfusion and improvement of fluid status and preload" occurred "after administration of general anesthetic agents and intravenous fluid administration during anesthesia induction."3
Michael O'Reilly, MD, EVP of Medical Affairs at Masimo, stated; "These studies confirm what previous results have shown—that PVI's accuracy at predicting fluid responsiveness is better than traditional measurements and similar to the newer invasive monitoring techniques at fraction of the cost and complexity. Because PVI is noninvasive and is available from the same sensor used with Masimo Rainbow SET® Pulse CO-Oximeters, it has the strong potential to expand and improve fluid monitoring and management in patients who would otherwise not be monitored with the newer invasive techniques."
PVI is available as part of Masimo Rainbow SET Pulse CO-Oximetry™—the first-and-only technology platform to noninvasively measure blood constituents and fluid responsiveness that previously required invasive procedures, including: noninvasive and continuous total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI, in addition to the Measure-Through Motion and Low-Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI).
1 Y. Kawashima, Y. Shiraishi, S. Sato. Does the Pleth Variability Index correlate with stroke volume variation?. Eur J Anaesthesiol 2009; 26 (Suppl 45): 3AP5-9
2 K. Takeyama, M. Yoshikawa. The change of upper limbs PVI in spinal block (The comparison in high spinal block and non-high spinal block). Eur J Anaesthesiol 2009; 26 (Suppl 45): 3AP5-1
3 J. Mizuno, Y. Morita, A. Kakinuma, S. Morita. Perfusion Index and Pleth Variability Index after administration of general anesthetic agents. Eur J Anaesthesiol 2009; 26 (Suppl 45): 4AP8-3
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpHb and PVI will provide a sufficient level of sensitivity and specificity to facilitate continuous measurement capabilities and deliver clinical improvement over alternative hemoglobin and fluid assessment measurement capabilities to allow for further adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. Flo Trac is a registered trademark of Edwards Lifesciences.


Masimo Debuts New Rad-8® Pulse Oximeter to Largest Gathering of Sleep Specialists from Around the World at SLEEP 2009
New Features and Design Optimize Care and Process Efficiencies for Clinicians in both the Sleep Center and Homecare Environments
Irvine, California – June 9, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today the debut of its new compact, lightweight Rad-8 pulse oximeter at SLEEP 2009, the 23rd Annual Meeting of the Associated Professional Sleep Societies (APSS), June 6-11, 2009 in Seattle, Washington. Newly redesigned with greater clinical efficiency in mind, the new Masimo Rad-8 has a streamlined look and a host of easy-to-use special features that enable sleep clinicians to more effectively and efficiently monitor, diagnose, and care for patients with sleep disorders.
According to the National Institutes of Health (NIH), more than 70 million Americans are affected by chronic sleep disorders. Sleep apnea is a very common yet serious sleep disorder where a person's breathing is uncontrollably and repeatedly interrupted during sleep, resulting in very brief or prolonged periods of oxygen deprivation. Serious problems can result from the oxygen deprivation of sleep apnea—including: heart disease, high blood pressure and learning/memory problems—and, if left untreated, sleep apnea can be life-threatening.1
Pulse oximetry is the standard method for assessing oxygenation in sleep testing and, as such, plays a critical role in treatment decisions, including whether to administer Continuous Positive Airway Pressure (CPAP) therapy or perform surgery. Inaccurate pulse oximetry data can have serious implications because false pulse oximetry desaturations can lead to misdiagnosis and inappropriate treatment or surgery, while missed true desaturations can prevent correct diagnosis and potentially lifesaving treatment. The new Rad-8 combines the unmatched sensitivity and specificity of Masimo SET® Measure-Through Motion and Low-Perfusion pulse oximetry—clinically-proven to reduce false alarms by over 90% and increase capture of true desaturation events by 98%—with enhanced functionality to help clinicians better capture, analyze, and report vital oxygen saturation, pulse rate, and perfusion data for improved sleep disorder detection.
The superior fidelity of Masimo SET has been clinically-shown to outperform other pulse oximeters in the accurate identification and quantification of brief dips in oxygen saturation due to apneas and hypopneas—an important marker and measure of severity for Obstructive Sleep Apnea (OSA) diagnosis and treatment. In fact, previous research conducted at Montreal Children's Hospital in Quebec found that using a Masimo pulse oximeter with very short averaging time was not only more accurate in detecting true desaturation events, including brief dips in oxygen saturation as well as larger ones, but could also "significantly reduce workload and improve reliability of desaturation detection" over other pulse oximeters. Study findings confirmed that Masimo detected 98.6% of true desaturations, while the N-395 detected only 45.3%, leading researchers to conclude that "the sensitivity and motion artifact rejection characteristics of the Nellcor N-395 oximeter are not adequate for a pediatric sleep laboratory setting."2
And, based on clinician input, Rad-8 now features an intuitive user-interface and easy menu navigation to save time and enable faster, easier set-up, and operation, while one-touch quick access buttons allow clinicians to engage special features in an instant. New user-selectable alarm settings make it quick and easy for clinicians to set and save configurations for specific patient monitoring needs and unique clinical applications. In addition, 72-hour trending, configurable Sleep and Home modes, and enhanced data collection/reporting compatibility make the new Rad-8 the ideal patient monitoring solution for sleep center, home, sub-acute, and transport applications.
1 National Institutes of Health "Frontiers of Knowledge in Sleep and Sleep Disorders: Opportunities for Improving Health and Quality of Life." March 2004. http://www.nhlbi.nih.gov/about/ncsdr/research/research-a.htm
2 Robert Brouillette, MD, Jacinthe Lavergne, RRT, Andra Leimanis, BSc, Gillian Nixon, MB ChB, FRACP, Sylvia Ladan, RRT, Christine McGregor, RRT. "Differences in Pulse Oximetry Technology can Affect Detection of Sleep-Disordered Breathing in Children." Anesth Analg 2002; 94: S47-S53.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the commercial availability of the new Rad-8 will serve to increase adoption, market share and/or revenues for the company, risks that Masimo SET sensitivity and specificity performance will be duplicated in other studies and applications, risks related to our assumption that the new Rad-8 will enable clinicians to more effectively and efficiently monitor, diagnose, and care for patients with sleep disorders, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended April 4, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. Other trademarks used herein are the property of their respective owners.


Two New Clinical Studies Show That Limited Exposure to Blood Transfusion Significantly Increases Morbidity and Mortality After Surgery
Studies Advocate Blood Conservation and Appropriate Indicators for Transfusion
Irvine, California – June 8, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that two new studies - one conducted in patients undergoing general surgery and published in the Journal of American College of Surgeons and another conducted in patients undergoing cardiac surgery and published in the Anesthesia & Analgesia- provide additional new evidence that transfusion of just one or two units of blood significantly increases infection, pneumonia, sepsis, and mortality after surgery.1,2 These studies suggest that transfusions and their associated risks could be "largely avoided" through implementation of better blood management techniques and "more appropriate indicators" for transfusions.
Blood transfusions may be necessary to ensure survival when a patient is bleeding heavily or has severe symptomatic anemia. However, transfusions are also given in the presence of stable anemia or when significant blood loss is expected but does not occur. These two new studies add to the growing evidence that transfusions carry life-threatening risks and urge that in the absence of benefit from transfusion, avoidance of transfusions through the use of more restrictive transfusion practices could improve patient outcomes.
In the general surgery study, researchers evaluated 125,177 patients from 121 hospitals and showed that after adjusting for all risk variables, transfusion of a single unit of blood increased 30-day mortality by 32% and morbidity (pneumonia, sepsis, or surgical site infection) by 23%. Transfusion of two units of blood increased the mortality risk by 38% and morbidity risk by 40%. In the cardiac surgery study, researchers evaluated long-term survival of 9,079 patients at eight hospitals and showed that transfusion of one or two units of blood increased six-month mortality 67% and five-year mortality 16% .
"These two new studies demonstrate that the risk of blood transfusion is significant and thus we should avoid transfusions when ever possible," stated Dr. Aryeh Shander, Clinical Professor of Anesthesiology, Medicine and Surgery at Mt. Sinai School of Medicine in New York, NY. "The current practice of using intermittent, invasive measurements of hemoglobin to help guide transfusion decisions may contribute to unnecessary blood transfusions. Blood transfusion should not simply be based on any particular level of hemoglobin but rather a thorough evaluation of the patient, including whether hemoglobin levels are stable or changing. The ability to continuously and noninvasively trend a patient's hemoglobin level offers a breakthrough in blood management. Continuous and noninvasive SpHb™ monitoring has the potential to greatly improve clinical decision-making and reduce patient exposure to allogeneic transfusion, reduce complications, and preserve a precious resource and costs."
Masimo continuous and noninvasive hemoglobin monitoring (SpHb) technology is available as part of the upgradeable Masimo Rainbow SET® Pulse CO-Oximetry platform.
1 Bernard AC et al. Intraoperative transfusion of 1U to 2U of packed red blood cells is associated with increased 30-day mortality, surgical site infection, pneumonia, and sepsis in general surgery patients. Journal of the American College of Surgeons. 2009;208:931-937.
2 Surgenor SD et al, for the Northern New England Cardiovascular Disease Study Group. The Association of Perioperative Red Blood Cell Transfusions and Decreased Long-Term Survival After Cardiac Surgery. Anesthesia & Analgesia 2009; 108:1741-1746.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the accuracy and repeatability of the Capgemini results; risks related to our assumption that Masimo SpHb technology and products will provide faster, easier and safer means for measuring total hemoglobin and will deliver a sufficient level of clinical improvement over alternative measurement capabilities to enable more restrictive transfusion practices, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended April 4, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks,
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Leading University Hospital Completes System-wide Conversion to Masimo SET® Pulse Oximetry
Standardizing on Masimo SET Allows Clinicians to Overcome the Limitations of Conventional Pulse Oximetry, Offering Significant Clinical Advancements
Irvine, California—June 3, 2009 – Masimo, the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion Pulse Oximetry, today announced it has completed the conversion of Duke University Hospital to Masimo SET pulse oximetry technology.
"Ultimately, the decision to convert to Masimo SET came down to what was best for patients," said Tony Caruso, Senior Director of Clinical Engineering, Duke University Health System in Durham, North Carolina. "It is critically important for us to access technologies that result in improvements in patient care. In this case, we believe we have improved our ability to increase detection of true clinical events earlier."
"In the emergency department, there are many times when we don't have time to second-guess our pulse oximeter performance, wonder whether the measurements we are getting are accurate, or take excessive time to obtain a reading," said Frank DeMarco, RN, emergency department clinical operations director, Duke University Hospital. "This technology offers us improvements over the system we had used previously and, hopefully, will contribute to more effective clinical operations."
Masimo Founder and CEO, Joe E. Kiani, stated, "We are happy to be the pulse oximetry standard-of-care solution chosen by the Duke University Hospital system to noninvasively and continuously monitor their patients. With the unprecedented accuracy and performance of Masimo SET, clinicians at Duke are afforded the opportunity to better monitor, manage and treat patients using advanced pulse oximetry capabilities that allow them to detect life-threatening events earlier."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumption that Masimo SET and Masimo Rainbow SET will deliver a sufficient level of clinical improvement over alternative pulse oximetry and noninvasive patient monitoring solutions to allow for further adoption of the technology at other hospitals, risks related to our assumption that Duke University Hospital's system-wide conversion to Masimo technology will serve to substantially increase revenues, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. Other trademarks used herein are the property of their respective owners.


New NIH-Funded Study Shows Masimo Rainbow SET® May Help Clinicians Assess Oxygenation in Children with Sickle Cell Disease
Study Presented at the American Thoracic Society by Researchers at The Children's Hospital of Philadelphia Confirms Accuracy and Clinical Value of Noninvasive SpCO® and SpMet®
Irvine, California – May 19, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that a new independent clinical study, funded by the National Institutes of Health (NIH) and presented at the American Thoracic Society in San Diego, CA, shows that the noninvasive measurement of carboxyhemoglobin (SpCO) and methemoglobin (SpMet) with Masimo Rainbow SET Pulse CO-Oximetry is accurate and may help clinicians better detect hypoxemia—a potentially life-threatening lack of oxygen in the blood—in children with sickle cell disease.
Sickle cell disease is an inherited blood disorder characterized by abnormally-shaped red blood cells that compromise vital blood and oxygen supply to the tissues and organs, causing life-threatening complications. The presence of carboxyhemoglobin and methemoglobin in a sickle cell patient's blood reduces oxygen supply further because these dyshemoglobins that take the place of normal oxyhemoglobin lack the ability to carry any oxygen. However, conventional two-wavelength pulse oximeters are incapable of measuring carboxyhemoglobin and methemoglobin levels and as a result cannot provide clinicians with an accurate picture of their patients' true oxygenation status in the presence of dyshemoglobins.
Masimo Rainbow SET—the first-and-only technology that noninvasively and continuously measures SpCO and SpMet, in addition to total hemoglobin (SpHb™), oxygen content (SpOC™), PVI, oxyhemoglobin (SpO2), pulse rate, and PI—allows for faster and more accurate assessment of the patient's true oxygenation, which may help to advance the care and management of patients with sickle cell disease.
In the study presented, Dr. Caboot and colleagues at Children's Hospital of Philadelphia simultaneously compared SpCO and SpMet measurements obtained noninvasively using the Masimo Radical-7 Pulse CO-Oximeter with invasive blood draws and laboratory analysis of carboxyhemoglobin and methemoglobin in 38 asymptomatic children (2-18 years of age) with sickle cell disease. The researchers found SpCO (with a bias of 0.1% and precision of > 2.2%) and SpMet (with a bias of -0.19% and precision of > 0.39%) to be closely comparable to invasive laboratory measurements. Researchers concluded that the Masimo Radical-7 Pulse CO-Oximeter "is useful in measuring carboxyhemoglobin and methemoglobin levels in pediatric patients with sickle cell disease."
Masimo Executive Vice President of Medical Affairs, Dr. Michael O'Reilly, stated, "This research not only reinforces the clinical accuracy of SpCO and SpMet in assessing the true oxygenation of patients, but also highlights the importance of measuring these dyshemoglobins while managing and treating pediatric patients with sickle cell disease."
Caboot, J.B., Jawad, A.F., McDonough, J.M., Bowdre, C.Y., Ohene-Frempong, K., Smith-Whitley, K., Allen, J.L. "Non-Invasive Measurements of Carboxyhemoglobin and Methemoglobin in Pediatric Patients with Sickle Cell Disease." Am. J. Respir. Crit. Care Med., Apr 2009; 179: A4788. Presented at the American Thoracic Society (ATS) Annual Meeting, May 19, 2009, San Diego, California. http://www.abstracts2view.com/ats09/view.php?nu=ATS09L_2057
Editor's Note: The study abstract available online depicts study data and results at the time of the "call for abstracts" submission deadline, although the study was on-going and continued beyond the submission date. The data and results captured in this press release represent the updated, final study data and results presented at the ATS International Conference on May 19, 2009.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results and our belief that SpCO and SpMet will improve clinical assessments and the identification of hypoxemia in patients with sickle cell disease, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended April 4, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks,
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Study Funded by the National Institutes of Health (NIH) Finds Masimo SpCO® May Provide a Noninvasive Measure of Acute Asthma Severity in Children
Study Presented at the Pediatric Academic Societies Annual Meeting by Vanderbilt University Researchers Shows a Statistically-Significant Association between SpCO and Measures of Lung Function
Irvine, California – May 13, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that a new clinical study conducted in the Emergency Department at Vanderbilt Children's Hospital shows that the noninvasive measurement of carbon monoxide in the blood with Masimo Rainbow SET® Pulse CO-Oximetry (SpCO) may help clinicians better assess acute asthma severity during and after treatment. The study was funded by the National Institutes of Health (NIH) and presented at the Pediatric Academic Societies (PAS) Annual Meeting on May 5, 2009.1
Asthma is a life-threatening inflammatory disease of the airways that affects more than 6 million children in the U.S., leading to more pediatric hospitalizations than any other cause.2 However, a common challenge for children with asthma is the requirement of a forced expiratory test called spirometry. In contrast to spirometry, SpCO—a noninvasive measurement easily obtained from Masimo Rainbow SET Pulse CO-Oximeters and sensors already used in many hospitals—does not require patient instruction or breathing effort. As a result, SpCO may help to improve the assessment of asthma severity and response to treatment in young children and patients who are unconscious, heavily sedated, unable to understand and follow instructions, or have limitations that would interfere with vigorous respiratory efforts.
"We have limited measures to assess severity of acute asthma exacerbations and the finding of an association between carboxyhemoglobin (SpCO) by multi-wavelength Pulse CO-Oximeter may have clinical importance," stated Donald H. Arnold, MD, MPH, Associate Professor of Emergency Medicine and Pediatrics, Vanderbilt University School of Medicine, Nashville, Tennessee. "Our preliminary results suggest that SpCO is a measure of oxidative stress and inflammation in pediatric patients with acute asthma exacerbations."
Dr. Arnold and colleagues at Vanderbilt used the Masimo Radical-7 Pulse CO-Oximeter to measure SpCO in 139 children (5-10 years of age) during acute asthma exacerbations and 2-hours after initiation of corticosteroid and bronchodilator treatment. Comparing SpCO measurements to conventional measures of airway obstruction and inflammation, researchers found a significant association between SpCO and percent predicted Forced Expiratory Volume in 1 Second (%FEV1, p = 0.001) and airway resistance (p = 0.04), as well as a trend with exhaled nitric oxide (eNO, p = 0.1). Study findings showed that for every 6% increase in SpCO, there was an associated 79% proportionate decrease in lung function (%FEV1, p=0.015) and a trend indicating SpCO may predict lung function after 2-hours of treatment, as measured by the change in %FEV1 (p = 0.06). Researchers concluded that SpCO may "represent a noninvasive, effort-independent measure of acute asthma disease severity as assessed by physiologic measures."
Masimo Executive Vice President of Medical Affairs, Dr. Michael O'Reilly, stated, "This study adds new evidence that expands the value of Masimo's Pulse CO-Oximetry SpCO measurement. The researchers at Vanderbilt Children's Hospital have shown that the ability to easily and quickly perform SpCO measurements on almost any patient without cooperation or risk has the potential to enable more immediate, accurate, and reliable asthma severity assessment."
1 D. H. Arnold, T. Gebretsadik, D. Resha, T. V. Hartert. "Association of Carboxyhemoglobin Levels with Clinical Measures of Acute Asthma Severity." Presented at the Pediatric Academic Societies (PAS) Annual Meeting, May 5, 2009, Baltimore, Maryland. http://www.abstracts2view.com/pas/view.php?nu=PAS09L1_3201
2 Centers for Disease Control and Prevention. "Current Asthma Prevalence" 2005. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/ashtma03-05/asthma03-05.htm
Editor's Note: The study abstract available online via the PAS website depicts study data and results at the time of the "call for abstracts" and submission deadline (late 2008), although the study was on-going and continued beyond the submission deadline. The data and results captured in this press release represent the actual study data and results presented at the PAS Annual Meeting on May 5, 2009.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, and our belief that noninvasive SpCO measurements will prove to be an effective clinical indicator of lung function and acute asthma severity in children or other patients such that SpCO measurement can effectively substitute for spirometry, as well as other factors discussed in the "Risk Factors" section of our Annual Quarterly Report on Form 10-Q for the fiscal quarter year ended April 4, 2009, filed with the Securities and Exchange Commission ("SEC") on May 6, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended April 4, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks,
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at the Deutsche Bank 34th Annual Health Care Conference
IRVINE, Calif., May 12, 2009 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced that its management is scheduled to present at the Deutsche Bank 34th Annual Health Care Conference in Boston, Massachusetts, on Tuesday, May 19, 2009, at 2:35 p.m. EDT. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at http://www.masimo.com.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports First Quarter 2009 Financial Results
Q1 2009 Highlights:
- Earnings per share increased by 47% to $0.22 per share from $0.15 in prior year
- Product revenues increased approximately 25% to $74.5 million from $59.7 million in prior year
Irvine, California, May 5, 2009 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, today announced its financial results for the first quarter of 2009.
For the first quarter of 2009, Masimo reported product revenues of $74.5 million representing a 24.7% increase over $59.7 million for the first quarter of 2008. Including royalty revenues, Masimo reported total 2009 first quarter revenues of $85.5 million compared to $71.1 million for the first quarter of 2008. For the first quarter of 2009, Masimo reported earnings per share of $0.22 compared to $0.15 per common share for the first quarter of 2008.
Masimo reported that it shipped 27,700 Masimo SET and Masimo Rainbow SET oximetry units, excluding handheld units, during the first quarter of 2009, resulting in at least 587,000 of Masimo SET and Masimo Rainbow SET pulse oximeters in use worldwide. In the first quarter of 2009, revenues from Masimo Rainbow SET products increased to $3.1 million from $2.7 million in the same prior year quarter.
Joe E. Kiani, Chairman and Chief Executive Officer of Masimo, said, "Despite the continuing difficult economic environment, we are happy that our revenues and earning were better than we had expected in Q1. We believe that this performance is largely due to our gold standard Masimo SET pulse oximetry technology and our expanding Masimo Rainbow SET Pulse CO-Oximetry platform, including the March 23, 2009 commercial release of total hemoglobin, the world's first continuous non-invasive total hemoglobin monitor."
Masimo also reported that as of April 4, 2009, cash and cash equivalents totaled $152.2 million, up from $146.9 million at January 3, 2009.
Conference Call
Masimo will hold a conference call today at 2:00 p.m. PT (5:00 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation number for both dial-in numbers is 95073118. A live web cast of the conference call will be available online from the "Investor Relations" page of the Company's corporate web site at www.masimo.com. After the live web cast, the call will remain available on Masimo's website through June 5, 2009. In addition, a telephonic replay of the call will be available until May 19, 2009. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 95073118.
About Masimo
Masimo (NASDAQ: MASI) develops, manufactures and markets innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the Company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical and laboratory studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO® ), methemoglobin (SpMet®) and plethysmograph variability index (PVI®), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com. Any information contained in, or that can be accessed through, our website is not incorporated by reference into, nor is it in any way a part of, this release.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations, prospects and business generally; and expectations regarding our ability to design and deliver innovative new noninvasive technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo Rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any new products and technologies of ours; obtaining regulatory approval of our current and future products and technologies, including the recently announced total hemoglobin measurement; the risk that the implementation of our international realignment, even if timely implemented, will not produce the anticipated operational and financial benefits, including a lower effective tax rate; the loss of our customers the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website, at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
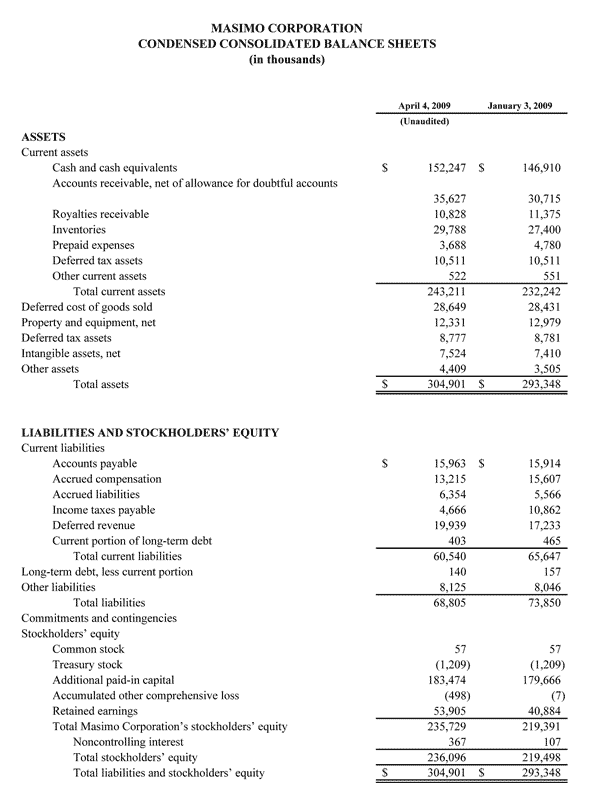



FEMA Adds Masimo Rad-57® Pulse CO-Oximeter™ to Required Medical Equipment List
Citing Need for Early and Accurate Carbon Monoxide Exposure Detection Following Disasters, Agency Unanimously Approves and Authorizes Funds to Purchase Rad-57's
Irvine, California – April 28, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that the Masimo Rad-57 Pulse CO-Oximeter has been added to the Federal Emergency Management Agency's (FEMA) list of required health and safety equipment for all Urban Search & Rescue (US&R) task forces throughout the United States. FEMA authorizes funding for each of its US&R task forces to purchase multiple Rad-57's, enabling them to quickly, accurately and noninvasively detect carbon monoxide (CO) poisoning on the scene of disaster recovery operations.
FEMA has 28 national US&R task forces staffed and equipped to conduct round-the-clock search and rescue operations following earthquakes, tornadoes, floods, hurricanes, aircraft accidents, hazardous materials spills, and catastrophic structure collapses. Search and rescue operations often function in confined or poorly ventilated spaces with gas-powered equipment producing elevated levels of CO, which until now had been an overlooked hazard. Adding the Rad-57 to its mandatory medical equipment cache now allows FEMA's US&R task forces to quickly, noninvasively evaluate and address the significant health and safety risks associated with CO poisoning for both civilians and rescue personnel in the urban search and rescue environment—helping to save and sustain lives, minimize suffering and enhance safety.
"Early and accurate CO exposure detection is important for successful mitigation and treatment of carbon monoxide poisoning," stated Neil Hampson, MD, Medical Director, Center for Hyperbaric Medicine at the Virginia Mason Medical Center in Seattle, Washington. "The Rad-57 will allow FEMA's US&R teams to swiftly, thoroughly and easily assess and diagnose CO poisoning to increase their public safety efforts in the urban search and response environment."
According to the Centers for Disease Control and Prevention (CDC), carbon monoxide—a common, yet lethal poison produced whenever any carbon-based fuel, such as gas, oil, kerosene, wood, or charcoal is burned—presents a significant two-fold risk during a disaster when "chemicals are most commonly released from businesses and industries, storage tanks, agricultural facilities and homes," and "when power outages occur, the use of alternative sources of fuel or electricity cause CO to build up." The most common symptoms of CO poisoning are headache, dizziness, weakness, nausea, vomiting, chest pain, and confusion, while high levels of CO inhalation can cause loss of consciousness and death. Unless suspected, CO poisoning can be difficult to diagnose because the symptoms mimic other illnesses.
Joe Kiani, Founder and CEO of Masimo, stated, "When elevated CO levels are present, disaster often strikes a second time, placing victims and their brave, unsuspecting rescuers in grave jeopardy. This is now a preventable disaster for rescuers and victims alike with Masimo Rad-57 in action on the front lines of U.S. disaster search and rescue efforts."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumption that the Rad-57 will deliver a sufficient level of sensitivity and specificity for improved CO detection over alternative methods to facilitate rapid diagnosis and treatment in the urban search and rescue environment, risks related to our belief that prior positive results and clinical outcomes achieved by the Rad-57 will be repeated in future incidents, and as well as other factors discussed in the "Risk Factors" section of our Annual Report on Form 10-K for the fiscal year ended January 3, 2009, filed with the Securities and Exchange Commission ("SEC") on March 4, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Annual Report on Form 10-K for the fiscal year ended January 3, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. Other trademarks used herein are the property of their respective owners.
NOTE TO REPORTERS AND EDITORS:
The CDC case report, titled: "Carbon Monoxide Kills Three Volunteer Firefighters Inside Well in Pennsylvania," available online at: http://www.cdc.gov/niosh/face/In-house/full9030.html, illustrates the significant tragedy and inherent risks associated with rescue attempts in confined or poorly ventilated area where dangerously elevated CO levels are present.


Capgemini Releases New Study on Financial Impact of Masimo Noninvasive and Continuous Hemoglobin (SpHb™)
Hospital-wide Implementation of Masimo Rainbow SET® Pulse CO-Oximetry™ Could Generate Nearly $500,000 in Net Annual Cost Savings and Financial Gains
Irvine, California – April 16, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that Capgemini, a leading supplier of global consulting and technology services, has released a study showing that a typical 500 bed hospital incorporating Masimo Rainbow SET Pulse CO-Oximetry into its clinical standards and care pathways could generate nearly $500,000 in net annual cost savings and financial gains.
Capgemini reported that significant financial benefits could be derived from incorporating noninvasive total hemoglobin (SpHb) by helping clinicians prevent unnecessary blood transfusions, identify internal bleeding earlier, and increase patient throughput. The study concluded that "whether considered on a per-patient, department, or hospital-wide analysis, there are significant clinical and financial benefits to implementing Pulse CO-Oximetry technology."
Masimo commissioned the Capgemini study for a third-party evaluation of the potential financial benefits of Masimo Rainbow SET. Hospital inputs were evaluated by Capgemini through 70 in-depth one-on-one interviews with clinicians and other decision-makers at 15 U.S. acute care hospitals and then quantified through a follow-on survey of 200 hospital-based physicians. The study found that the majority of anesthesiologists and two-thirds of surgeons believed that SpHb monitoring could prevent at least one unnecessary blood transfusion in every ten surgical cases on which it was used, contributing to $93,600 in net annual cost savings in a surgical department using 20 SpHb-enabled devices. The study also found that the majority of intensivists believed that SpHb monitoring could reduce intensive care length of stay by at least one day for every 15 or fewer patients on which it was used, contributing to $67,350 in net annual cost savings in an intensive care department using 10 SpHb-enabled devices.
Joe Kiani, Founder and CEO of Masimo, stated, "We believe that the Capgemini study will significantly aid hospitals seeking to evaluate the initial and ongoing investment in Masimo Rainbow SET by clearly identifying each of the various mechanisms by which SpHb can improve the clinical process of care and create net financial benefits."
The Capgemini study can be downloaded here. In addition, a customized financial analysis based on a hospital's own inputs incorporated into the Capgemini financial model is available upon request.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the accuracy and repeatability of the Capgemini financial model and results; risks related to our belief that the applications of Masimo Rainbow SET measurements described in the foregoing statements will deliver a sufficient level of clinical improvement over alternative measurement capabilities to create substantial financial benefits for hospitals, allow for rapid adoption of the technology, and materially increase market share and/or revenues for the company, as well as other factors discussed in the "Risk Factors" section of our Annual Report on Form 10-K for the fiscal year ended January 3, 2009, filed with the Securities and Exchange Commission ("SEC") on March 4, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal year ended January 3, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. Other trademarks used herein are the property of their respective owners.


Masimo to Report First Quarter 2009 Financial Results on May 5, 2009
Conference call and webcast to begin after markets close at 2:00 p.m. PT (5:00 p.m. ET)
Irvine, Calif., April 14, 2009—Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced it will release first quarter 2009 financial results for the period ended April 4, 2009 after the market closes on Tuesday, May 5, 2009.
A conference call to review the results will begin at 2:00 p.m. PT (5:00 p.m. ET) and will be hosted by Joe E. Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the Company's corporate web site at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation number for both dial-in numbers is 95073118. After the live webcast, the call will remain available on Masimo's website through June 5, 2009. In addition, a telephonic replay of the call will be available through May 19, 2009. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 95073118.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at http://www.masimo.com.
Masimo Corporation
Investor Contact
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Clinical Study Shows Masimo PVI™ Accurately Predicts Fluid Responsiveness in the ICU
First Study to Expand PVI Utility Beyond the OR Presented at the 29th International Symposium on Intensive Care and Emergency Medicine in Brussels
Irvine, California – March 31, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that a new independent clinical study demonstrates Masimo PVI to be an "accurate index of fluid responsiveness" for critical care patients in the intensive care unit (ICU).1 The study, presented at the 29th International Symposium on Intensive Care and Emergency Medicine on March 25, 2009, in Brussels, Belgium, is the first to show the potential value of Masimo PVI to predict fluid responsiveness beyond the operating room (OR) into the ICU.
Critically-ill patients are at great risk for volume depletion. Fluid administration is critical to optimizing oxygen delivery to organs and tissues, but giving too much fluid can induce life-threatening adverse effects. Therefore, parameters that aid clinicians in fluid management decisions may help improve patient outcomes. The most validated predictor of fluid responsiveness is pulse pressure variation (ΔPP). However, this parameter requires an invasive arterial pressure catheter, which is not appropriate for all patients, or special software, which is not available in all monitoring systems. Masimo PVI is not only noninvasive, enabling easy application on almost any patient, but is also easily obtained from existing or field upgradable Masimo Rainbow SET® pulse oximeters and sensors that are already being used to monitor SpO2 and pulse rate.
In the study, Marc Feissel, MD, and colleagues at Le Centre Hospitalier Belfort-Montbeliard (CHBM) in Belfort, France, along with a team of researchers from Assistance Publique–Hôpitaux de Paris (AP-HP) in Le Kremlin-Bicetre, France, conducted a two-step analysis of 43 mechanically-ventilated patients with septic shock to: 1) identify the optimal Masimo PVI threshold for distinguishing fluid responders from non-responders; and 2) test the accuracy of PVI at the optimal threshold to predict fluid responsiveness.
In the first step, researchers infused fluid (500 ml saline) in with pulse pressure variation (ΔPP) ≥ 15% and performed passive leg raising in patients with ΔPP <15% while simultaneously recording Masimo PVI and ΔPP. A >15% increase in velocity-time-integral (VTI) from echocardiography was used to determine fluid responsiveness. After comparing all data for the 25 enrolled ICU patients in the first step, researchers found that a "threshold PVI value of 20 identified patients with ΔPP >15% with a sensitivity of 84% and specificity of 90%."
In the second step, researchers found that a PVI value of 20 was an effective threshold for discriminating fluid responders from non-responders among 18 additional ICU patients, with PVI >20 predicting all 8 fluid responders and PVI < 20 predicting all non-responders. The researchers concluded that a PVI value of 20 "distinguished responders from non-responders with good sensitivity and specificity," and appeared to represent an "accurate index of fluid responsiveness" for critically-ill patients in the ICU.
Masimo Executive Vice President of Medical Affairs Dr. Michael O'Reilly, stated: "We are pleased to see the value and significance of Masimo PVI in the ICU, where clinicians are often faced with difficult decisions about whether to administer fluid. While traditional invasive measurements offer limited accuracy at predicting fluid responsiveness, this study showed that a PVI threshold of 20 was able to accurately predict both fluid responders and non-responders, underscoring the strong potential for PVI to aid clinicians in optimizing fluid management and improving patient outcomes."
1 M. Feissel, R. Kalakhy, J. Badie, G. Robles, J. Faller, JL. Teboul. "Plethysmography Variability Index: A New Fluid Responsiveness Parameter." Presented at the 29th International Symposium on Intensive Care and Emergency Medicine (ISICEM) Annual Meeting, March 25, 2009, Brussels, Belgium. http://ccforum.com/content/13/S1/P205
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that PVI will prove to be an effective clinical indicator of patient hydration and the need for fluid loading, and will deliver a sufficient level of clinical improvement over alternative fluid assessment methods to allow for rapid adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our Annual Report on Form 10-K for the fiscal year ended January 3, 2009, filed with the Securities and Exchange Commission ("SEC") on March 4, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Annual Report on Form 10-K for the fiscal year ended January 3, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. Other trademarks used herein are the property of their respective owners.


Masimo Initiates Full Market Release of First-Ever Noninvasive Continuous Hemoglobin Monitor
Success in Limited Market Release Hospitals Highlights Early Clinical Benefits and Patient Impact
Irvine, California – March 24, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that it has initiated the full market release of its breakthrough noninvasive and continuous hemoglobin (SpHb™) monitoring technology. As the first noninvasive and continuous hemoglobin monitoring technology to receive FDA 510(k) clearance and be available for widespread commercial adoption, Masimo SpHb is already transforming the way hemoglobin testing is performed at over 40 hospitals in the U.S., Europe, Asia, and Africa, that have participated in the technology's limited market release initiated in September of 2008.
The availability of noninvasive, continuous, and immediate hemoglobin measurements is expected to have wide ranging clinical impact, from surgery and intensive care to less acute care settings, including the emergency department, physician office, ambulatory surgery center, and long-term care facility by facilitating prompt detection of internal bleeding and more appropriate administration of blood transfusions. Early benefits and impact of Masimo SpHb were evident in feedback received from clinicians at hospitals around the world who participated in the limited market release.
Ronald Miller, MD, Chief of Anesthesia, Professor and Chairman of the Dept. of Anesthesia and Perioperative Care at the University of California, San Francisco, stated, "Masimo SpHb is an impressive new tool that helps us to more safely guide patients in surgery through to recovery. With it, not only can we spot hemoglobin changes as they occur, but we can see where they are heading. This ability to identify an upward or downward hemoglobin trend on a second-by-second basis as it occurs has been of tremendous value."
Randy Marcel, MD, Medical Director and Chief of Anesthesiology at The Heart Hospital Baylor Plano in Plano, Texas, stated, "In the past, we've only received glimpses of our patients' hemoglobin levels from lab measurements, but now we have complete and real-time hemoglobin visibility. We initially purchased SpHb for use during cardiac surgeries in the OR, and brought in additional units for the ICU, where post-operative monitoring of internal bleeding is critical to patient recovery."
Javed Akhtar, MD, FAAP, Medical Director, Pediatric Intensive Care Unit at Creighton University Medical Center in Omaha, Nebraska, stated, "We purchased the SpHb monitor after seeing it in a hands-on demonstration, and I'm glad we did! SpHb has reduced the traumatic experience for pediatric patients, increased the satisfaction of parents, and reduced the workload on our nursing staff, phlebotomist and laboratory personnel."
Madhava Karunarathna, MD, OB/GYN at Balangoda Hospital in Sri Lanka, stated, "With SpHb, we now have accurate hemoglobin measurements available at our fingertips, around the clock. In cases of severe hemorrhaging during and after childbirth, SpHb has enabled us to immediately identify and continuously assess blood loss severity to better manage internal bleeding, prevent overloading of fluid, and decrease maternal death."
Adi Abdussalam Adham, MD, Chief Manager at Accidents Hospital Abu Saleem in Tripoli, Libya reinforced Dr. Karunarathna's comments, adding that: "In the operating room, Masimo SpHb has enabled us to more effectively monitor blood loss and better manage transfusions in surgery, while routine screening in the ED has helped us to more rapidly identify patients with anemia or internal bleeding."
Bertrand Debaene, MD, Anesthesiologist at the University Hospital Center of Poitiers in Poitiers, France believes the combination of SpHb and PVI may prove to have significant clinical benefits, saying that "SpHb, along with PVI, have been important improvements for both the department and our patients. The ability to track hemoglobin and fluid volume in real-time allows us to be more precise in our clinical routine."
SpHb is part of the Masimo Rainbow SET Pulse CO-Oximetry patient monitoring platform—the first-and-only upgradable technology platform capable of continuously and noninvasively measuring multiple blood constituents and helping to predict fluid responsiveness in patients previously requiring invasive procedures. Masimo Rainbow SET noninvasive measurements—including: total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI™, oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI)—have the potential to facilitate faster, easier and safer health decisions.
Currently available in bedside Masimo Radical-7® and Rad-87® Pulse CO-Oximeter patient monitors, SpHb will also be offered in handheld monitors and select multiparameter patient monitoring brands through Masimo Rainbow SET Pulse CO-Oximetry technology license agreements.
Joe Kiani, Founder and CEO of Masimo said, "SpHb's success during the limited market release phase at hospitals around the world illustrates its expected significant impact on clinical practice and patient care. In addition and as we expected, our limited market release program provided us with the opportunity to enhance the robustness in low perfusion situations and broaden the cross section of individuals on which the technology performs. The ability for clinicians in hospitals, private practices, and other healthcare settings to perform immediate and continuous noninvasive hemoglobin monitoring creates new opportunities to save lives, optimize decision making, improve care, and reduce costs."
SpHb has received regulatory clearance in the U.S., Canada, Europe and Australia, and is now available for sale in most of the countries in the world.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the commercial availability of SpHb technology and products will serve to increase adoption, market share and/or revenues for the company, risks that limited market release clinical results may not be duplicated in the full market launch, risks related to our assumption that SpHb technology and products will provide faster, easier and safer means for measuring total hemoglobin and will lead to additional device sales, and risks related to our assumptions that Rainbow measurements will deliver a sufficient level of clinical improvement over alternative capabilities to allow for rapid adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our Annual Report on Form 10-K for the fiscal year ended January 3, 2009, filed with the Securities and Exchange Commission ("SEC") on March 4, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Annual Report on Form 10-K for the fiscal year ended January 3, 2009, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. Other trademarks used herein are the property of their respective owners.


New Clinical Study Finds Masimo Rainbow SET® Pulse CO-Oximetry™ Accurate in the Noninvasive Monitoring of Fluid Status During Surgery
UCSF Researchers Presented Study Data Demonstrating the Unique Ability of Masimo PVI™ to Reflect Acute Changes in Intravascular Fluid Volume at the International Anesthesia Research Society
83rd Scientific Congress
Irvine, California – March 16, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low-Perfusion pulse oximetry, today announce that a new clinical study, independently conducted by researchers from the University of California-San Francisco (UCSF), demonstrates that Masimo PVI accurately and reliably reflects acute changes in intravascular fluid volume (preload).1 The study, presented at the International Anesthesia Research Society (IARS) 83rd Scientific Congress on March 14th in San Diego, Calif., affirms PVI as a highly predictive indicator of patient fluid status.
Assessing whether a patient needs fluid to increase their cardiac index (amount of blood the heart pumps each minute) is one of the biggest challenges anesthesiologists face during surgery. Although fluid administration is critical to optimizing patient status and enabling end organ preservation, unnecessary fluid administration is associated with increased morbidity and mortality2 and traditional invasive measurements are only 50 to 60% accurate at predicting improvement in cardiac index after volume administration.3 PVI—a new method for noninvasive and automatic assessment of fluid responsiveness—has been shown in multiple studies to predict fluid responsiveness in mechanically ventilated patients, helping clinicians to optimize fluid administration and improve patient outcomes.4-6
In the study, Errol P. Lobo, M.D., PhD, at UCSF in San Francisco, Calif., and colleagues used Masimo Radical-7 Pulse CO-Oximeters to noninvasively measure and continuously track PVI, perfusion index (PI) and pulse rate (PR) data during 16 consecutive liver transplant operations. Researchers extracted and analyzed the noninvasive data at three critical points where preload changes occur rapidly: 1) immediately before clamping, 2) during clamping, and 3) immediately after clamping of the inferior vena cava (IVC), and found that PVI changed rapidly and significantly in response to known acute changes in preload (clamping and unclamping of IVC) in 100% of the cases.
The study results showed that PVI increased in response to IVC clamping (from 11.4, +/- 4.3, to 25.2, +/- 4.4; p<0.0001) and decreased after unclamping (from 25.2, +/- 4.4, to 8.9, +/- 3.8; p<0.0001) in all 16 transplant patients. Researchers concluded that this study data demonstrates that PVI may have "a role in monitoring intravascular volume in mechanically ventilated patients."
PVI is available as part of Masimo Rainbow SET Pulse CO-Oximetry—a revolutionary noninvasive patient monitoring platform that measures multiple blood constituents and helps to predict fluid responsiveness in patients previously requiring invasive procedures. The first-and-only upgradable noninvasive blood constituent monitoring technology platform capable of continuously and noninvasively measuring total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and pleth variability index (PVI™), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), Masimo Rainbow SET has the potential to facilitate faster, easier and safer health decisions.
Masimo Executive Vice President of Medical Affairs Dr. Michael O'Reilly, stated: "This UCSF study adds to the growing evidence base demonstrating PVI to be an accurate and reliable indicator of fluid status and responsiveness in mechanically-ventilated patients under general anesthesia. The ability to noninvasively and automatically assess fluid responsiveness in surgical patients has the potential to increase patient safety, improve the quality of care, and reduce the cost of care for hospitals and surgery centers."
1 E. Lobo, C. Niemann, P. Talke. Anesthesia and Perioperative Medicine, University of California-San Francisco, San Francisco, California. "Effect of Preload Changes on Pleth Variability Index During Liver Transplants." Available online at: http://www.abstractsonline.com}
2 Michard F, Teboul JL. "Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence." Chest. 2002 Jun;121(6):2000-8.
3 Joshi G. "Intraoperative Fluid Restriction Improves Outcome After Major Elective Gastrointestinal Surgery." Anesthesia Analgesia 2005; 101:601-5.
4 Cannesson M, Desebbe O, Rosamel P, Delannoy B, Robin J, Bastien O, Lehot JJ. "Pleth variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre." British Journal of Anaesthesia 2008; 0:aen133v1-7.
5 Christopher Wray, M.D., Jack Buckley, M.D., Derek Kwan, B.S., Tayeba Maktabi, Aman Mahajan, M.D., Ph.D. Anestheiology, UCLA David Geffen School of Medicine, Los Angeles, California. "Ability of Pleth Variability Index to Detect Preload Changes in Orthotopic Liver Transplant Patients."
6 Olivier Desebbe, M.D., Bertrand Delannoy, R.A., Jean-Jacques Lehot, M.D., Ph.D., Olivier Bastien, M.D., Ph.D., Maxime Cannesson, M.D. Department of Anesthesiology, Louis Pradel Hospital, Lyon-Bron, France. "Pleth Variability Index: A Noninvasive Device for Fluid Responsiveness Assessment during Anesthesia."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; that PVI will prove to be an effective clinical indicator of patient hydration and the need for fluid loading, and will deliver a sufficient level of clinical improvement over alternative fluid assessment methods to allow for rapid adoption of the technology; and our belief that the applications of Masimo Rainbow SET measurements described in the foregoing statements will deliver a sufficient level of clinical improvement over alternative measurement capabilities to materially increase market share and/or revenues, as well as other factors discussed in the "Risk Factors" section of our Annual Report on Form 10-K for the fiscal year ended January 3, 2009, filed with the Securities and Exchange Commission ("SEC") on March 4, 2009, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. Other trademarks used herein are the property of their respective owners.


Masimo Reports Fourth Quarter and Full Year 2008 Financial Results
Record results mark 22nd consecutive quarter and 14th year of consecutive product revenue growth. Company also announces international realignment to better support growing non-US business.
Q4 2008 Highlights:
- Product revenues increased 29.4% to $71.4 million
- Rainbow revenues increased 161% to $4.7 million
- Over 31,700 Masimo SET and Masimo Rainbow SET pulse oximeter units were shipped in Q4 2008
- International realignment results in a Q4 2008 tax charge of $0.25 per share and a Q4 2008 net loss of $0.01 per share; Q4 2008 earnings per share, excluding the tax charge, was $0.24 per share
Full Year 2008 Highlights:
- Product revenues increased 29.7% to $258.9 million
- Rainbow revenues increased 91% to $13.4 million
- Over 120,000 Masimo SET and Masimo Rainbow SET pulse oximeter units were shipped in 2008
- International realignment results in a Q4 2008 tax charge of $0.25 per share and full year 2008 earnings per share of $0.53; full year 2008 earnings per share, excluding the Q4 tax charge, was $0.78 per share
Irvine, California, March 3, 2009 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced its financial results for both the quarter and year ended January 3, 2009.
For the fiscal fourth quarter, Masimo reported product revenues of $71.4 million representing a 29.4% increase over $55.2 million for the fourth quarter of 2007. Including royalty revenues, Masimo reported total 2008 fourth quarter revenues of $83.1 million compared to $69.3 million for the fourth quarter of 2007. For the fourth quarter, Masimo reported a loss of $0.01 per share, resulting from a $14.9 million, or $0.25 per share, tax charge related to the implementation of its new international business organization and structure. Excluding this tax charge, fourth quarter net income would have been $14.4 million, or $0.24 per diluted share, compared to $0.20 per common share for the fourth quarter of 2007. In addition, higher research and development tax credits added $0.03 per share in the fourth quarter of 2008 and $0.02 in the fourth quarter of 2007. Masimo also reported that it shipped 31,700 Masimo SET and Masimo Rainbow SET oximetry units, excluding handheld units, during the fourth quarter of 2008, up from 29,400 in the comparable prior year period, resulting in a new estimated worldwide installed base of 567,000 Masimo SET and Masimo Rainbow SET pulse oximeters.
During the fourth quarter of 2008, Masimo implemented a new international business organization and structure designed to better serve and support Masimo's growing international business. By centralizing its international operations, including sales management, marketing, customer support, planning, logistics and administrative functions, Masimo believes it will be able to develop a more efficient and scalable international organization – capable of being even more responsive to the business needs of its international customers - all under one centralized management structure. As result of this realignment, in Q4 2008, Masimo incurred a one-time tax charge related to expenses for sharing in the costs of our ongoing research and development efforts as well as the prepayment of licensing commercial rights to utilize pre-existing intangibles. As a result of this new international business structure, Masimo believes that its future income tax rates will decline although future income tax rates will depend on various factors, including profits (losses) before taxes, changes to tax law and the geographic composition of pre-tax income.
For the fiscal year ended January 3, 2009, Masimo reported product revenues of $258.9 million, up 29.7% from $199.7 million in 2007. Including royalty revenues, Masimo's total revenues were $307.1 million for the year ended January 3, 2009, up from $256.3 million in 2007. For the year ended January 3, 2009, Masimo reported earnings of $31.9 million or $0.53 per share, including the $14.9 million tax charge related to the implementation of its new international business organization and structure. Excluding the Q4 2008 tax charge, net income would have been $46.8 million, or $0.78 per share compared to $0.60 per share in 2007. In the year ended January 3, 2009, Masimo shipped 120,100 Masimo SET and Masimo Rainbow SET pulse oximeter units, excluding handheld pulse oximeters, compared to 116,300 in 2007.
In the fourth quarter of 2008, revenues from Masimo Rainbow SET products increased to $4.7 million or 161% from $1.8 million in the same prior year quarter. For the year ended January 3, 2009, revenues from Masimo Rainbow SET products were $13.4 million, an increase of 91% compared to 2007. As a result, 2008 Masimo Rainbow SET revenues accounted for 5.2% of total product revenues up from 3.7% in 2007.
Joe E. Kiani, Chairman and Chief Executive Officer of Masimo, said, "We have been fortunate that, despite the very challenging economic environment, hospitals and physicians continue to focus on the quality and efficiency of their patients' care and, as a result, continue to migrate to Masimo's gold standard SET pulse oximetry and Rainbow SET Pulse CO-Oximetry platforms. Our on-going business model, which is based on both new product innovation and a recurring revenue stream, is continuing to show resilience in this difficult worldwide economic climate."
As of January 3, 2009, cash and cash equivalents totaled $146.9 million, up from $96.7 million at December 29, 2007, including the previously announced first quarter 2008 repayment in full of a $26.7 million debt obligation which had been established in early fiscal 2007.
Financial Guidance
While providing financial guidance in this economic environment is more difficult than in prior periods, Masimo expects 2009 product revenues will be between $310 million and $315 million. Masimo also expects 2009 royalty revenues to be between $42 million to $46 million. As a result of these ranges, Masimo expects total 2009 revenues to be between $352 million and $361 million. For the full year 2009, Masimo expects its GAAP earnings per share to be between $0.83 and $0.87 per common share. Included in these 2009 earnings per share ranges are an expected reduction in year over year royalty payments, an expected increase in 2009 non-cash stock based compensation expenses of $11.9 million, up from $7.7 million in 2008 and the expected benefits from our new international business structure. The projections and guidance set forth above are estimates only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 2:00 p.m. PT (5:00 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation number for both dial-in numbers is 84029998. A live web cast of the conference call will be available online from the "Investor Relations" page of the Company's corporate web site at www.masimo.com. After the live web cast, the call will remain available on Masimo's website through April 3, 2009. In addition, a telephonic replay of the call will be available until March 17, 2009. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 84029998.
Non-GAAP Measures
Masimo prepares its consolidated financial statements in conformity with accounting principles generally accepted in the United States of America, or U.S. GAAP. In an effort to provide investors with additional information regarding the Company's results and to provide a meaningful period-over-period comparison of the Company's financial performance, the Company uses non-GAAP financial measures as defined by the Securities and Exchange Commission. The differences between the U.S. GAAP and non-GAAP financial measures are reconciled below. In presenting comparable results, the Company discloses non-GAAP financial measures when it believes such measures will be useful to investors, analysts and other interested parties in evaluating the Company's underlying business performance on a comparable basis with past and future reported earnings per share and with the financial guidance provided in this release. Management uses the non-GAAP financial measures to evaluate the Company's financial performance against internal budgets and targets. Importantly, management believes non-GAAP financial measures should be considered in addition to, and not in lieu of, U.S. GAAP financial measures. These non-GAAP financial measures are not based on a comprehensive set of accounting rules or principles and may be different from non-GAAP financial measures used by other companies.
About Masimo
Masimo (NASDAQ: MASI) develops, manufactures and markets innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the Company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical and laboratory studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO® ), methemoglobin (SpMet®) and plethysmograph variability index (PVI®), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com. Any information contained in, or that can be accessed through, our website is not incorporated by reference into, nor is it in any way a part of, this release.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations, prospects and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and expectations for total revenues, including royalty revenues, product revenues and Rainbow revenues, non-cash stock based compensation charges, GAAP earnings per share and non-GAAP pro forma earnings per share for the full fiscal year 2009. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo Rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any new products and technologies of ours; obtaining regulatory approval of our current and future products and technologies, including the recently announced total hemoglobin measurement; the risk that the implementation of our international realignment, even if timely implemented, will not produce the anticipated operational and financial benefits, including a lower effective tax rate; the loss of our customers, the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008 filed with the Securities and Exchange Commission ("SEC") on October 29, 2008, which you may obtain for free on the SEC's website, at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Mark P. de Raad
Executive Vice President, Finance and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
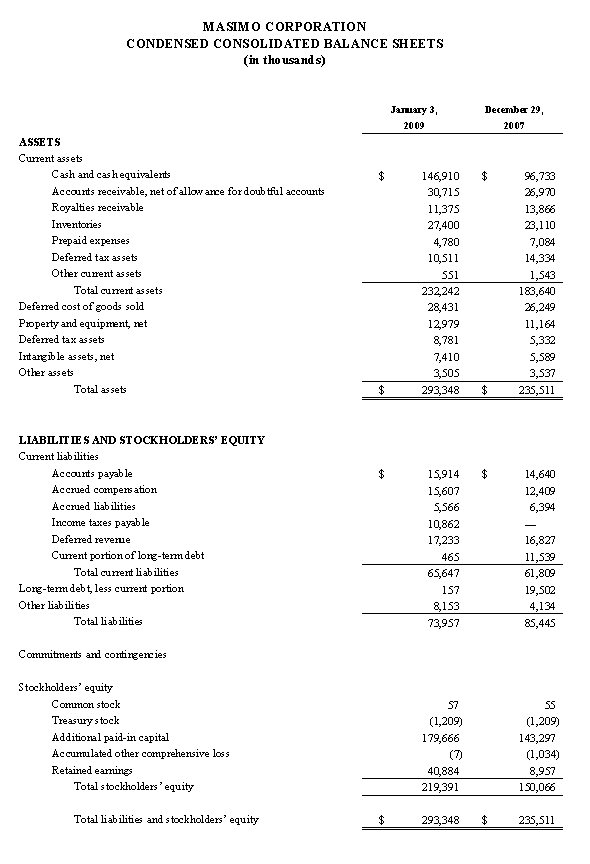
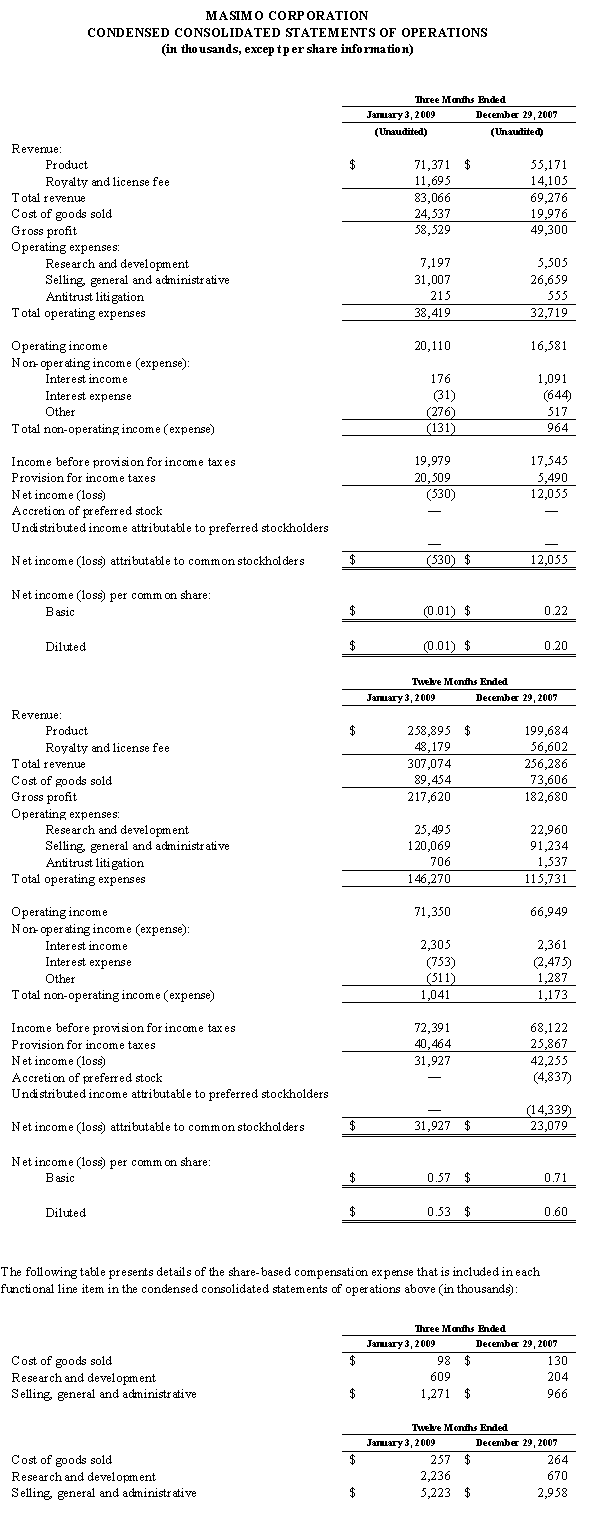



Three Healthcare GPOs to Offer Masimo Rainbow SET®Pulse CO-Oximetry™ to Their Members
Novation, MedAssets Supply Chain Systems, and Amerinet Also Extend Existing Multi-Year Supplier Agreements
Irvine, California – March 2, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, announced today that three of the nation's top hospital Group Purchasing Organizations (GPOs) have established a new Pulse CO-Oximetry™ category and extended multi-year supplier agreements for Masimo SET®pulse oximetry and Masimo Rainbow SET®Pulse CO-Oximetry products. Masimo licenses the technology and supplies Pulse CO-Oximetry products that noninvasively measure multiple blood constituents, which previously required invasive blood drawing procedures.
Establishing a new Pulse CO-Oximetry product category on national contract portfolios allows Novation, MedAssets Supply Chain Systems, and Amerinet to offer an innovative new selection of patient monitoring devices clinically proven to help clinicians provide earlier detection of potentially life-threatening conditions. This enables customer hospitals and healthcare facilities to purchase breakthrough noninvasive blood monitoring products that may increase patient safety, improve the quality of care, and reduce the cost of care at a great value.
Mark Miriani, President,MedAssets Supply Chain Systems, stated: "MedAssets remains focused on the important trends and issues facing the healthcare community and we recognize the importance of providing advanced technology solutions and innovative new product offerings to our customers. Pulse CO-Oximetry has the potential to improve patient safety and clinical performance, thereby delivering greater value to both hospitals and patients. We are proud to be among the first to offer Masimo Rainbow SET Pulse CO-Oximetry products on contract to our customers, creating the opportunity for measurable performance improvement as well as cost savings."
Brad Morgan, Director of Material Management, The Heart Hospital Baylor Plano, stated: "We are pleased that our GPO is making medical technologies like Masimo Rainbow SET available."
Masimo Rainbow SET Pulse CO-Oximetry is a noninvasive monitoring platform that measures multiple blood constituents and helps to predict fluid responsiveness in patients previously requiring invasive procedures. The first-and-only upgradable technology platform capable of continuously and noninvasively measuring total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and pleth variability index (PVI™), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), Masimo Rainbow SET has the potential to facilitate faster, easier and safer health decisions.
Martin Allard, M.B.Ch.B, FRC, Anesthesiologist, Loma Linda University Medical Center, stated: "Pulse CO-Oximetry has enormous potential. We chose the Masimo Rainbow SET Pulse CO-Oximetry platform because it provides us with the unique ability to noninvasively, continuously and immediately measure a variety of blood constituents, in addition to standard pulse oximetry measurements. Having this amount of vital physiologic data available at our fingertips has allowed us to make more rapid diagnoses, initiate intervention and treatment earlier, and make better care decisions for patients."
Rick Fishel, Masimo President of Americas and Worldwide OEM Business, stated: "Novation, MedAssets, and Amerinet are leading the way toward a new paradigm of potentially more effective and efficient patient care enabled by state-of-the-art noninvasive medical devices. These new GPO agreements reinforce the clinical and operational adoption of Masimo Rainbow SET Pulse CO-Oximetry as an emerging standard of care."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET®Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that creation of a new GPO product category for Pulse CO-Oximetry will serve to broaden the use of Masimo Rainbow SET Pulse CO-Oximetry products or provide substantially increased revenues, and risks related to our assumptions that Rainbow measurements will deliver a sufficient level of clinical improvement over alternative capabilities to allow for rapid adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, filed with the Securities and Exchange Commission ("SEC") on October 29, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. Other trademarks used herein are the property of their respective owners.


United Kingdom House of Commons Safety Group Recommends Carbon Monoxide Screening by Pulse CO-Oximetry™ to Reduce Misdiagnosis and Prevent Deaths
New Evidence from London Ambulance Service Study Shows Five Masimo Rad-57 Pulse CO-Oximeters™ Detected 83 Unsuspected CO Poisonings in One Year—Over a Third of the UK Reported Annual CO Poisonings
Irvine, California – February 18, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced that in a newly published report, the UK House of Commons All Party Parliamentary Gas Safety Group (APPGSG) recommends handheld, noninvasive Pulse CO-Oximeters for Emergency Departments, primary care providers, and medical professionals who visit patients' homes as a way to improve the country's rate of detection and diagnosis of carbon monoxide (CO) poisoning.1 In addition, new evidence from a London Ambulance Service pre-hospital CO screening study shows that using the Masimo Rainbow SET Rad-57 Pulse CO-Oximeter significantly improved detection of CO poisoning at an earlier stage, while reducing unnecessary hospital transports and patients entering the Accidents & Emergency system.2
The UK House of Commons APPGSG report, 'Raising Medical Professionals' Awareness of Carbon Monoxide Poisoning,' cites "increasing concern that little is being done to ensure that medical professionals are able to correctly diagnose the symptoms of carbon monoxide and treat victims effectively," as the catalyst that ultimately resulted in the group's January 2009 report and recommendations for action. The UK National Health Service (NHS) reports that hundreds of people are seriously injured or killed by CO poisoning in the UK each year.3 However, the APPGSG suggests that because of the lack of adequate testing, the actual number may be much higher. In addition, because the vast majority of cases simply go to their GP complaining of symptoms similar to the flu, the incidents of misdiagnosis by medical professionals are grossly under-reported.
"From the collated data, information gained during and prior to the feasibility study and correspondence with other interested agencies it is clear that there are gaping holes in the way that CO poisoning is recognised, monitored, treated, recorded and publicised in the United Kingdom," stated Andrew Humber, Team Leader, London Ambulance Service.
While authors of the APPGSG report advise that "targeting improved diagnosis of CO poisoning is doubly important not just in containing the immediate effects of the illness, but also in reducing the need to direct resources toward tackling subsequent chronic or acute medical problems," a year-long feasibility study also released in January 2009 by the London Ambulance Service demonstrates Masimo Rad-57's ability to deliver these benefits.
In their report, 'A Feasibility Study into Pre-Hospital Carbon Monoxide Monitoring of Patients,' the London Ambulance Service equipped three First Response Units (FRU) and two Hazardous Area Response Team (HART) vehicles with Rad-57s to determine the benefits of pre-hospital CO monitoring and found that the ability to noninvasively screen patients on the scene made an important difference in six key ways:
- Earlier detection & treatment of CO poisoning
- Prevention of further harm, possible higher level intoxications & long-term effects of untreated exposure
- Reduced clinical risk to the ambulance service & increased safety for the attending crew
- Improved on-scene triage ensures the fastest & most appropriate care (A&E or Hyperbaric)
- Reduction in unnecessary hospital transports & patients entering the A&E system
- Increased efficiency gains as ambulances/crews returned to core duties earlier
Prior to this study, London Ambulance crews had no way to accurately diagnose CO poisoned patients. With just five Rad-57s, the London Ambulance Service was able to diagnose and treat 83 patients with unsuspected CO poisoning in a year, which is over a third of the reported annual number of CO poisonings in the UK. "On site monitoring of carbon monoxide blood levels mean that the paramedics are now able to triage patients at scene and receive expert advice at the scene, so that they take patients to the most appropriate A&E department and those with severe CO poisoning can now be referred directly to appropriate hyperbaric units," stated Andrew Humber. "It is due to this that there has been a marked improvement in the time to treatment."
Masimo Founder and CEO, Joe E. Kiani, stated: "The UK recommendations and London Ambulance Service study are a call to action for all medical and emergency professionals who see patients with potential carbon monoxide poisoning. We are proud that the technology we have invented and produced is helping these professionals improve and save lives."
1 United Kingdom, House of Commons, All Party Parliamentary Gas Safety Group. "Raising Medical Professionals' Awareness of Carbon Monoxide Poisoning." January 2009. http://www.gisg.org.uk
2 London Ambulance Service, NHS Trust. "A Feasibility Study into Pre-Hospital Carbon Monoxide Monitoring of Patients." January 2009.
3 NHS Choices, "Carbon Monoxide Poisoning" http://www.nhs.uk/conditions/carbon-monoxide-poisoning/Pages/Introduction.aspx?url=Pages/what-is-it.aspx
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the positive results and clinical outcomes achieved in the London Ambulance Service study will be repeated in other studies, and risks related to our assumption that the Masimo Rad-57 will deliver a sufficient level of sensitivity and specificity for improved carbon monoxide detection over alternative methods to allow for rapid adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, filed with the Securities and Exchange Commission ("SEC") on October 29, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at the Roth Capital Partners 21st Annual OC Growth Stock Conference
Irvine, California – February 6, 2009 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced that its management is scheduled to present at the Roth Capital Partners 21st Annual OC Growth Stock Conference at The Ritz-Carlton, Laguna Niguel in Dana Point, California, on Wednesday, February 18, 2009, at 11:00 a.m. PT. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Clinical Study Presented at the Society for Critical Care Medicine Shows Value of Pulse CO-Oximetry™ to Identify Acquired Methemoglobinemia
1 out of 7 HIV+ Patients Receiving Dapsone Therapy at Significant Health Risk Due to Dangerously High Levels of Methemoglobin
Irvine, California – February 6, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced that a new study presented at the Society for Critical Care Medicine 38th Critical Care Congress suggests providers should consider routine screening with Masimo Rainbow SET Pulse CO-Oximetry for the immediate and noninvasive detection of potentially life-threatening acquired methemoglobinemia in patients receiving Dapsone therapy. Results showed that "Dapsone therapy is associated with a clinically significant elevation of methemoglobin levels" in 1 out of 7 HIV+ patients.
In the study, Hollis R. O'Neal, M.D., and colleagues at the Vanderbilt University School of Medicine in Nashville, TN, found that 32% of HIV positive patients receiving chronic Dapsone therapy, a commonly used antimicrobial agent for the treatment of prophylaxis of pneumocystis jirovecii pneumonia (PCP), had elevated methemoglobin blood levels (>2%) and 15% had dangerously high methemoglobin blood levels (>3%) when compared to a control group of HIV positive patients not receiving Dapsone.1 Dapsone has been shown to cause potentially deadly levels of methemoglobin—a damaged form of hemoglobin that cannot bind to oxygen—to accumulate in the blood. Elevated methemoglobin concentration reduces oxygen delivery to vital organs and tissues, which can lead to brain and organ damage, or even death.2 The researchers concluded: "As noninvasive Pulse CO-Oximetry is now available, providers should consider the routine screening of persons receiving Dapsone for methemoglobinemia."
Masimo Executive Vice President of Medical Affairs, Dr. Michael O'Reilly, stated: "Previous studies have shown that methemoglobinemia can occur in any patient type in any care area of the hospital. This study reinforces the need for clinicians to quickly, easily and conveniently screen all patients receiving Dapsone therapy using Masimo Rainbow SET Pulse CO-Oximetry as the first line of defense and prevention against acquired methemoglobinemia."
1 Hollis R. O'Neal, John H. Newman, Catherine McGowan, Stephen P. Raffanti, Timothy Sterling, Vanderbilt University School of Medicine, Nashville, TN. "The Prevalence of Methemoglobinemia in HIV-Infected Patients on Chronic Dapsone Therapy for Prophylaxis of Pneumocystis Jirovecci Infection." Society of Critical Care Medicine, February 2, 2009.
2 Ash-Bernal, R., Wise, R., Wright, SM. "The Johns Hopkins Study: Acquired Methemoglobinemia." Medicine (Baltimore). 2004 Sep;83(5):265-73.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the positive results and clinical outcomes achieved in this study will be repeated in other studies, and risks related to our assumption that Masimo Rainbow SET Pulse CO-Oximetry technology and devices will deliver a sufficient level of sensitivity for improved methemoglobinemia detection over alternative methods to allow for rapid adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, filed with the Securities and Exchange Commission ("SEC") on October 29, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report Fourth Quarter and Fiscal Year 2008 Financial Results on March 3, 2009
Conference call and webcast to begin after markets close at 2:00 p.m. PT (5:00 p.m. ET)
Irvine, California – February 5, 2009 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced it will release fourth quarter and fiscal year 2008 financial results for the period ended January 3, 2009 after the market closes on Tuesday, March 3, 2009.
A conference call to review the results will begin at 2:00 p.m. PT (5:00 p.m. ET) and will be hosted by Joe E. Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the Company's corporate web site at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation number for both dial-in numbers is 84029998. After the live webcast, the call will remain available on Masimo's website through April 3, 2009. In addition, a telephonic replay of the call will be available through March 17, 2009. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 84029998.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at http://www.masimo.com.
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Files Patent Infringement Suit Against Philips
Irvine, California – February 3, 2009 –Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low-Perfusion pulse oximetry, today announced that it has filed a patent infringement suit against Philips Electronics North America Corporation and Philips Medizin Systeme Böblingen Gmbh related to Philips FAST pulse oximetry technology and certain Philips patient monitors. The suit was brought in the United States District Court for the District of Delaware. Two patents at issue in this suit, related to Masimo's measure-through-motion technology, were successfully enforced in a previous suit by Masimo against Nellcor.
Masimo Founder and CEO, Joe E. Kiani, stated: "Given that Philips and Masimo have a long-term business relationship, we have patiently tried to resolve our differences regarding Philips' infringement of Masimo's patents without seeking the courts' intervention. Unfortunately, since our best efforts have not been successful, we believe we have no choice but to rely on the courts to resolve this dispute."
Masimo and Philips continue to be engaged in a long-term OEM agreement. Masimo intends to comply with the terms of the agreement, continuing to provide Philips with Masimo SET® technology for integration into Philips monitoring products, to the benefit of both companies' mutual customers.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors. Important factors that may cause such a difference for Masimo in connection with our litigation with Philips include, but are not limited to: our ability to prevail in the lawsuit against Philips, including any appeals; the ability of our patents to protect our intellectual property and products; our ability to enforce our intellectual property rights; and the risks associated with litigation in general, including the costs and time that must be devoted to litigation, the potential diversion of the attention of management and key employees that may result from being engaged in litigation, and the possibility of adverse results. Additional factors that may cause Masimo's actual results to differ materially from those expressed in forward-looking statements include, but are not limited to: those factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, filed with the Securities and Exchange Commission ("SEC") on October 29, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Steve Moran, Executive Vice President, General Counsel & Secretary
Masimo Corporation
949-297-7015
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Multi-Center Study Finds Masimo SET Pulse Oximetry Screening Significantly Improves Detection of Congenital Heart Disease in Newborns
Study Published in British Medical Journal Indicates Masimo SET May Also Reduce the Need for Pre-Operative Neonatal Intensive Care and Prevent Long-term Neurological Morbidity
Irvine, California – January 30, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that a new multi-center study of 39,821 newborns was published in the January 2009 issue of the British Medical Journal (BMJ). When compared to a cohort group of 108,604 newborns in whom no pulse oximetry screening was used, the study found that the addition of Masimo SET pulse oximetry screening before discharge increased detection of Congenital Heart Disease (CHD) by 28% (from 72% to 92%). In addition, researchers noted that "no baby died from undiagnosed duct dependent circulation" in the Masimo SET group, while five babies from the cohort group died during the same period.1
In the study, Anne de-Wahl Granelli, M.D., of the Queen Silvia Children's Hospital in Goteborg, Sweden, and colleagues screened babies born between 2004 and 2007 at five well baby nurseries using Masimo SET pulse oximetry. A positive pulse oximetry result was defined as an oxygen saturation of <95% measured both preductally (right hand) and postductally (either foot), or a measurement difference >3% between the two measurements. The study found that the positive predictive value of pulse oximetry screening was at least seven times higher than the best-case scenario for standard neonatal physical examination alone (20.7% vs. 3.1%).
Undiagnosed CHD presents devastating long-term morbidity and mortality risks, with 10-30 percent of the babies who die from CHD remaining undiagnosed until autopsy.2 Additionally, study authors noted that the rise in the proportion of babies who leave the hospital with undiagnosed critical CHD had multiple contributing factors, such as earlier discharge from maternity wards, the growing prevalence of babies rooming with their mothers instead of observational nurseries and reductions in pre-discharge neonatal clinical exams.
The researchers concluded that Masimo pulse oximetry screening of "all well babies in maternity units is practically feasible with a minimum use of nursing time" and that it "significantly improves" detection of duct dependent CHD. Researchers also commented that "the low false positive rate, the fact that other important pathology is unearthed by the screening and the likely reduced need for preoperative neonatal intensive care suggest that such screening will be cost effective."
The current study adds to previous research conducted by Dr. Granelli, which demonstrated that when compared to conventional pulse oximetry, only Masimo SET pulse oximetry is accurate and reliable for improving the detection of critical CHD.3
Masimo Executive Vice President of Medical Affairs Dr. Michael O'Reilly, stated: "Undiagnosed CHD frequently places the lives of newborns in grave danger. To ensure that these newborns are given the absolute best chance at survival, pre-discharge CHD detection and diagnosis is critical. The comprehensive population and protocol data gathered by these researchers demonstrate that the superior sensitivity and specificity of Masimo SET pulse oximetry technology is key to substantially increasing the ability to accurately diagnose CHD before newborns are discharged from the hospital."
1 Anne de-Wahl Granelli, et al., "Impact of Pulse Oximetry Screening on the Detection of Duct Dependent Congenital Heart Disease: a Swedish Prospective Screening Study in 39,821 newborns." BMJ. 2009; 338;a3037.
Available online at: http://www.bmj.com/cgi/content/full/338/jan08_2/a3037
2 Bonnet D, Coltri A, Butera G, Fermont L, Le Bidois J, Kachaner J, et al., "Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality." Circulation 1999;99:916-8.
3 Anne de-Wahl Granelli, et al., "Screening for Duct-dependent Congenital Heart Disease with Pulse Oximetry: A Critical Evaluation of Strategies to Maximize Sensitivity.." Acta Paediatrica. 2005; 94:1590-1596.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET® Pulse CO-Oximetry™, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the positive results and clinical outcomes achieved in this study will be repeated in other studies, and risks related to our assumption that Masimo pulse oximetry technologies and devices will deliver a sufficient level of sensitivity for improved CHD detection over alternative methods of newborn screening to allow for rapid adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, filed with the Securities and Exchange Commission ("SEC") on October 29, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


MedAlliance signs agreement with Masimo to provide an innovative offering of Masimo SET® pulse oximetry and Masimo Rainbow SET® Pulse CO-Oximetry™ products
(LONDON, ON): MedAlliance, Canada's newest and most flexible healthcare Group Purchasing Organization, is pleased to announce the signing of an agreement with Masimo, the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, that provides Members across Canada with tremendous value on a comprehensive and innovative offering of pulse oximetry and Pulse CO-Oximetry products.
The agreement gives MedAlliance Members immediate and direct access to Masimo's entire product offering, featuring industry-leading patient monitoring technologies that may enable clinicians to provide earlier detection and treatment of potentially life-threatening conditions to help increase patient safety, improve the quality of care, and reduce the cost of care. This includes Masimo SET® pulse oximeters, Masimo Rainbow SET® Pulse CO-Oximeters, as well as Masimo's full array of noninvasive sensors and advanced patient monitoring solutions.
"We are very pleased to partner with Masimo to provide savings to our membership," notes Leslie McGill, President of MedAlliance. "The purchase of capital equipment represents a critical, but significant expenditure for hospitals. The purpose of MedAlliance is to provide more choice and flexibility in purchasing, while also helping Canadian healthcare organizations achieve lower supply costs. This pulse oximetry agreement with Masimo will help us achieve our goal."
This new agreement marks the very first MedAlliance contract in the pulse oximetry category and joins a growing roster of multi-source, choice-based contracts, which will give healthcare providers savings in a wide range of contract areas including capital equipment, specialty medical/surgical products, medical implants and devices, maintenance, operations and repair supplies, and laboratory supplies.
The MedAlliance pulse oximetry contract with Masimo is now in place for Q4 purchases.
"We are proud to partner with MedAlliance in establishing a 'gold standard' pulse oximetry supply chain agreement built on the Masimo SET and Masimo Rainbow SET foundation of clinical, operational and patient safety excellence," says Masimo Founder and CEO, Joe E. Kiani. "This agreement allows MedAlliance member hospitals, health authorities and non-profit healthcare organizations throughout Canada to access advanced Masimo pulse oximetry technology and emerging Pulse CO-Oximetry standards of care at a great value."
About Masimo's Pulse Oximetry Offering
Masimo offers an extensive selection of pulse oximetry and Pulse CO-Oximetry solutions, including handheld and bedside devices; reusable and single patient use sensor options; remote monitoring and clinician notification systems; as well as its proprietary SatShare interface, which allows for a simple upgrade of an existing multi-parameter monitor to Masimo SET performance. And, since Masimo SET is the leading Measure-Through Motion and Low Perfusion SpO2 solution in more than 100 multi-parameter monitors and 50 monitoring brands worldwide, hospitals can easily integrate Masimo technology into any clinical setting.
Masimo Rainbow SET Pulse CO-Oximetry is a breakthrough noninvasive monitoring platform that measures multiple blood constituents and helps to predict fluid responsiveness in patients previously requiring invasive procedures. The first-and-only upgradable technology platform capable of continuously and noninvasively measuring total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and pleth variability index (PVI™), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), Masimo Rainbow SET has the potential to facilitate faster, easier and safer health decisions.
Masimo's newest noninvasive innovation, SpHb, allows healthcare professionals to instantaneously and continuously measure a patient's hemoglobin level in real-time to potentially detect chronic or acute anemia faster, identify occult bleeding earlier, and more effectively manage blood transfusions. With Masimo SpCO and SpMet, clinicians can quickly and accurately determine a patient's blood level of carboxyhemoglobin and methemoglobin—two critical dyshemoglobins proven to increase morbidity and mortality in a broad range of clinical settings.1-2 And, Masimo PVI is a new method for the noninvasive, automatic quantified measurement of dynamic changes that may help clinicians assess fluid responsiveness in patients.
1 Kao, et al., "Carbon Monoxide Poisoning. " Emergency medicine Clinics of North America. 22 (2004), pp. 985-1018
2 Ash-Bernal R, et al. "Acquired Methemoglobinemia A Retrospective Series of 138 Cases at 2 Teaching Hospitals." Medicine Sept 2004;83(5):265-273.
About the Partners
MedAlliance Supply Chain Services Corporation is Canada's newest and most flexible healthcare Group Purchasing Organization. A wholly owned subsidiary of Medbuy Corporation, MedAlliance is designed to assist Canadian hospitals in managing their supply chain cost through a wide variety of choice-based contracting services that maximize savings in the categories of capital equipment; specialty medical/surgical products; medical devices and implants; maintenance, operations and repair supplies; and laboratory supplies. Together with our partners, MedAlliance is changing the face of healthcare procurement in Canada.
Medbuy Corporation is Canada's leading healthcare Group Purchasing Organization. We deliver the best net price, at the lowest cost, of any Canadian healthcare GPO while securing value-added benefits that grow our Members' knowledge base. Medbuy is guided by the principles of contract commitment and compliance, while offering a comprehensive offering of national single-source contracts for healthcare supplies, pharmaceuticals and services. Maximizing supply chain savings means Medbuy Member hospitals can redeploy critical dollars where it counts – to patient care. For more information, visit www.medbuy.ca.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumption that entering into a multi-year agreement with MedAlliance will serve to broaden the use of Masimo SET pulse oximetry and Masimo Rainbow SET Pulse CO-Oximetry technologies or will serve to provide substantially increased revenues for the company, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, filed with the Securities and Exchange Commission ("SEC") on October 29, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
For further information, please contact:
Leslie McGill
President
MedAlliance Supply Chain Services Corporation
519-652-1688
Dana Banks
Masimo Corporation
949-297-7348
Medbuy is a registered trademark of Medbuy Corporation. MedAlliance is a trademark of MedAlliance Supply Chain Services Corporation.
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. CPT is a registered trademark of the American Medical Association.


Clinicians at Dartmouth-Hitchcock Medical Center Present New Data Showing Masimo Rainbow SET® Oximetry and Patient SafetyNet™ Improves Patient Outcomes and Reduces the Cost of Care
Presentation at the Society for Technology in Anesthesia Annual Meeting Quantifies Decreases in Rescue Calls and ICU Transfers with Improved ICU Utilization
Irvine, California – January 20, 2009 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that a team of clinicians at Dartmouth-Hitchcock Medical Center (DHMC) presented new outcome data from their year-long implementation study showing Masimo Rainbow SET Pulse CO-Oximetry and Patient SafetyNet remote monitoring and clinician notification system helps improve patient outcomes and reduce the cost of care by decreasing rescue calls and ICU transfers, resulting in improved ICU utilization. Study findings were presented at the Society for Technology in Anesthesia (STA) Annual Meeting in San Antonio, Texas on January 15, 2009.
Clinical data tracked by Dr. George T. Blike, Medical Director of Patient Safety, and his team at DHMC during the 12-month study period showed that, after the implementation of Masimo Rainbow SET and Patient SafetyNet, there was a 70% reduction in rescue calls and a 48% reduction in ICU transfers. In addition, the team found that the reduction in ICU transfers helped to free up an estimated 163 ICU-days annually for its 36-bed post-surgical unit, thereby increasing ICU access for more critically ill patients. The findings and data also indicate that there is a measurable cost advantage associated with Masimo Patient SafetyNet's ability to assist clinicians in the identification of the onset of patient distress earlier—preventing codes, rescues, and ICU transfers—contributing to significant decreases in patient care costs on the general care floor.
Financial data presented by the team showed that the cost of care for patients undergoing bilateral or multiple major joint prosthesis operations of the lower extremity who did not deteriorate to the point of rescue was 60% less ($19,987) than the cost of care for patients who did ($49,602). Additionally, the length of stay for non-rescued patients was 68% shorter than that of rescued patients (3.9 days versus 12.3 days). For major joint replacement operations of the lower extremity, the length of stay for patients who did not require a rescue call was 38% shorter (3.6 days versus 5.8 days), which resulted in a 31% decrease in their cost of care ($13,648 versus $19,765).
George T. Blike, M.D., Medical Director of Patient Safety at DHMC, stated; "The data tracked in this 12-month study demonstrates that routine patient monitoring on the general care floor using Masimo Rainbow SET and the Patient SafetyNet system, with its ability to measure oxygen saturation continuously and directly page a nurse when problems are identified, was associated with statistically significant reductions in the need for urgent and emergent interventions. Initial analysis shows we reduced the need for ICU transfers and, we believe, the cost of care through early detection and intervention of physiologic abnormalities. These results are such that we have expanded implementation of the system to two additional post-surgical units."
The Masimo Patient SafetyNet system combines Measure-Through Motion and Low Perfusion performance of Masimo Rainbow SET Pulse CO-Oximetry technology with wireless clinician alerts via pager to help provide a new level of safety to patients on general care floors, where nurse-to-patient ratios preclude the level of direct surveillance required and recommended to preempt adverse events. When physiologic parameters deteriorate, Masimo Patient SafetyNet system provides accurate, actionable alarm notification directly to a qualified clinician—enabling immediate and early intervention.
The growing frequency of unrecognized adverse incidents and avoidable patient safety issues resulting in death or serious physical injury is a preventable and costly human tragedy that the Journal of the American Medical Association has reported may account for 2.4 million extra hospital days, over 32,500 deaths, and $9.3 billion in excess charges in the United States annually.1 These startling statistics have prompted the convergence of leading healthcare accreditation and industry groups toward a common patient safety standard of continuous, noninvasive physiologic monitoring linked to an appropriate clinician alert and notification system for perioperative patients.
The Joint Commission's National Patient Safety Goals call for the "improved recognition and response to changes in a patient's condition" and recommend "a suitable method that enables health care staff members to directly request additional assistance from a specially trained individual(s) when the patient's condition appears to be worsening."2 The Anesthesia Patient Safety Foundation (APSF) recommendations advise healthcare professionals to "give consideration to the potential safety value of continuous postoperative monitoring of oxygenation and ventilation in at-risk patients without delay," urging that "it is critical that any monitoring system be linked to a reliable process to summon a competent healthcare professional to the patient's bedside in a timely manner."3 The American Society of Anesthesiologists (ASA) Clinical Practice Guidelines have established that "continuous pulse oximetry monitoring in a step-down unit reduces the likelihood of perioperative complications among patients who are believed to be at increased perioperative risk from OSA."4
Masimo Founder and CEO, Joe E. Kiani, stated; "By revolutionizing pulse oximetry with the invention of Signal Extraction Technology (SET), we created a new era where pulse oximeters could measure through motion and low perfusion, resulting in the virtual elimination of false alarms with an increased ability to detect true events. The clinical community and Masimo collectively hoped that our technology could help caring clinicians improve the quality of care for their patients. In the last ten years, we have witnessed our technology being used to help save the eyesight of neonates, detect congenital heart defects in newborns and save countless lives. The evidence gathered by Dr. Blike and his team at Dartmouth-Hitchcock Medical Center continues to illustrate the substantial impact of Masimo technology on patient safety benchmarks. These caring clinical professionals have done their hospital and the medical community a great service by implementing a solid protocol with Masimo Rainbow SET and Patient SafetyNet to more effectively and efficiently care for their patients after surgery on the general care floor. Their results provide a compelling case and opportunity for hospitals to improve healthcare outcomes while decreasing costs."
A study published in the Journal of Critical Care Medicine estimates that there are approximately 820,000 unmonitored beds in non-critical care settings in the U.S.
1 The Journal of the American Medical Association (JAMA). Excess Length of Stay, Charges, Mortality Attributable to Medical Injuries During Hospitalization, 2003.
2 The Joint Commission: "Facts about the 2008 National Patient Safety Goals"; June 1, 2007.
http://www.jointcommission.org/facts_about_the_national_patient_safety_goals/
3 Anesthesia Patient Safety Foundation (ASPF) Initiatives: "Safety During Patient-Controlled Analgesia"; October 13, 2006.
http://www.apsf.org/initiatives_safety.php
4 American Society of Anesthesiologists (ASA): "Practice Guidelines for the Perioperative Management of Patients with Obstructive Sleep Apnea"; Anesthesiology 2006;104:1081-93.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI™, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumption that the positive results and clinical outcomes presented by Dartmouth-Hitchcock Medical Center will be repeated at other hospitals, risks related to our assumption that Masimo Patient SafetyNet will deliver a sufficient level of clinical improvement over alternative remote monitoring systems to allow for rapid adoption of the technology on general care floors, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, filed with the Securities and Exchange Commission ("SEC") on October 29, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. CPT is a registered trademark of the American Medical Association.
