News & Media-2008
Home / 2008
2008
NEWS & MEDIA:

Masimo Rainbow SET® SpCO® and SpMet® Measurements Receive CPT Codes and Medicare Reimbursement
Irvine, California – December 23, 2008 – Masimo, the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that noninvasive carboxyhemoglobin (SpCO®) and methemoglobin (SpMet®) will have CPT coding and pricing under the 2009 Medicare Clinical Laboratory Fee Schedule, effective January 1, 2009.1
The new CPT codes for SpCO (88740) and SpMet (88741) will allow healthcare providers to bill and receive up to $7.33 per day when testing eligible patients. Medicare will only allow a single payment per patient/per day for either SpCO or SpMet, regardless of how many tests are performed on that date. Medicare will not make payment for both SpCO and SpMet for the same patient on the same date of service.
1 2009 Medical Clinical Laboratory Fee Schedule. Available at http://www.cms.hhs.gov
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the establishment of CPT codes for Masimo SpCO and SpMet will increase the application and broaden the use of Masimo Rainbow SET Pulse CO-Oximetry technology or serve to provide substantially increased revenues for the company, and risks related to our assumptions that SpCO and SpMet will deliver a sufficient level of clinical improvement over alternative measurement capabilities to allow for rapid adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, filed with the Securities and Exchange Commission ("SEC") on October 29, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation. CPT is a registered trademark of the American Medical Association.

Masimo to Present at the 27th Annual J.P. Morgan Healthcare Conference
IRVINE, Calif., December 23, 2008 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that its management is scheduled to present at the 27th Annual J.P. Morgan Healthcare Conference at the Westin St. Francis Hotel in San Francisco, CA on Monday, January 12, 2009, at 2:00 p.m. PT. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (Nasdaq: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at http://www.masimo.com.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Happy Holidays from Masimo
Irvine, California (December 22, 2008)—This has been an exciting year for us here at Masimo, as we continue to see clinicians use our technologies to improve patient safety and enhance the quality of patient care in hospitals, doctor's offices, and EMS/Fire departments around the world. It is at this very special time of year that we look to share our blessings by making a special donation on your behalf to a charitable organization that needs our help the most.
As we look to the future, we are encouraged by the successes of the past and driven by the desire to continue working with the clinical community to facilitate even greater advancements in patient care in the years to come. It is with great appreciation for our customers, partners, shareholders and employees that we close out this year by making a donation of $10 to the charity of your choice. We will make this donation in the name of each person who is an official member of Livewire as of today and who responds to this Livewire with his or her charity choice from the list below:
- Amnesty International
- Opportunity International
- CARE
- Swan Foundation in Medical Ethics
- Doctors Without Borders
- UNICEF
- Huntington's DSA
- United Way
- Make-a-Wish Foundation
- World Vision
- March of Dimes
- 911 Research

Please send us an e-mail specifying your selection to: charity@masimo.com. Only requests by e-mail to this address will be processed. We also encourage you to include any comments or suggestions you might have that will help us to better fulfill our mission and adhere to our guiding principles.
Masimo Mission Statement
Improving patient outcomes and reducing cost of care by taking noninvasive monitoring to new sites and applications.
Masimo Guiding Principles
Remain faithful to your promises and responsibilities.
Thrive on fascination and accomplishment and not on greed and power.
Make each day as fun as possible.
Strive to make each year better than the year before, both personally and for the Team.
Do what is best for patient care.
Thank You!

AARC Presentation Cites Masimo Patient Safety Net as Key Factor in "Improving Patient Outcomes and Reducing Costs" in Post-Acute Care Facilities
Masimo also receives the AARC Zenith Award for Equipment and Service Excellence at the 54th Annual International Respiratory Congress in Anaheim
Irvine, California – December 16, 2008 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion and Low-Perfusion pulse oximetry, announced that the clinical benefit of its remote monitoring and clinician notification system, Masimo Patient SafetyNet, was featured in a presentation titled Global Perspectives of Facility-Prolonged Mechanical Ventilation Care at the American Association of Respiratory Care (AARC) 54th Annual International Congress in Anaheim, California, on December 15, 2008.
Gene Gantt, RRT and Manager of Business Development for Linde Respiratory Support Services (RSS), a leader in post-acute respiratory care, presented how weaning patients off ventilator care in a post-acute care setting provides improved outcomes and reduced costs. According to Mr. Gantt, a major challenge to weaning patients off ventilator care in the post-acute environment was a safe and easy-to-use patient surveillance system that could help clinicians preempt adverse events. Mr. Gantt reported that the integration of the Masimo Patient SafetyNet system into Linde's Knoxville, TN facility protocol has been a significant factor in successfully implementing their weaning protocol and achieving a 75% wean rate with an average length-of-stay of only 40 days. The initiative has been so successful at improving the quality of life for patients and reducing the overall cost of care that Linde-RSS has decided to expand use of the Patient SafetyNet system to both their Memphis and Chattanooga facilities, as well.
"Using the Masimo Patient SafetyNet System not only raised the bar in terms of the quality of care that could be delivered in a post-acute setting—proving to be the right thing to do for patient safety—it is also paying for itself through clinical efficiency," Mr. Gantt explained. "Since our clinicians are no longer wasting their time chasing false oximetry alarms, they are freed up to focus on patient care."
In addition to the Patient SafetyNet presentation and the continued excitement generated by Masimo's noninvasive and continuous hemoglobin monitoring technology (SpHb), the company announced that it received the AARC's prestigious Zenith Award. More than 400 companies were eligible for one of five 2008 Zenith Award honors presented. The Zenith Award is AARC's top industry recognition honoring respiratory care equipment and pharmaceutical manufacturers for quality and service excellence. Award recipients are chosen via special ballot by the association's membership of more than 44,000 respiratory care professionals based upon the quality of its equipment and/or supplies, the accessibility and helpfulness of its sales personnel, as well as the company's responsiveness, service record, truth in advertising, and overall support of the respiratory care profession.
Masimo Founder and CEO, Joe E. Kiani, stated, "We are delighted to see the results of Linde RSS's use of Masimo SET and Patient SafetyNet and are honored that AARC members have chosen to recognize Masimo for the superior performance, service, and integrity that Masimo provides to the clinical community. Respiratory clinicians were among the first to embrace the performance of Masimo SET pulse oximeters 10 years ago, but they often faced challenges in gaining access to Masimo's pulse oximetry technology. It is gratifying to see that despite the challenges, so many hospitals and clinicians now have access to Masimo SET and the benefits that our technology brings to their day-to-day patient care. It is our pleasure to serve the respiratory care profession in their mission in caring for the critically ill."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET Pulse CO-Oximetry continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
About the AARC
The American Association for Respiratory Care, headquartered in Dallas, Texas, is a professional association of respiratory therapists that focuses primarily on respiratory therapy education and research. The organization's goals are to ensure that respiratory patients receive safe and effective care from qualified professionals as well as supporting respiratory health care providers. The association continues to advocate on behalf of pulmonary patients for appropriate access to respiratory services provided by qualified professionals. Further information about the AARC may be found at www.AARC.org.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumption that this award will serve to increase market acceptance and adoption of Masimo technologies and products or substantially increase revenues, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, filed with the Securities and Exchange Commission ("SEC") on October 29, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Nihon Kohden Expands Integration of Masimo SET® Pulse Oximetry Technology Worldwide
Agreement with Japan's leading medical equipment provider broadens availability of Masimo SET to the global clinical community
Irvine, California – November 18, 2008 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, and Nihon Kohden, one of the largest patient monitoring companies in the world, jointly announce an expanded technology licensing agreement that calls for the integration of Masimo SET into Nihon Kohden bedside and patient monitoring products worldwide.
"Growing clinical preference for Masimo SET continues to drive pulse oximetry technology selection for our customers," stated Kazuo Ogino, Chairman and CEO of Nihon Kohden. "We began integrating Masimo SET into Nihon Kohden products in the U.S. and Canada in 2006. The demand for Masimo technology worldwide continues to grow and we want to provide our customers with their preference of pulse oximetry technology. I believe the collaboration between Masimo and Nihon Kohden will create a great opportunity for our customers who look for an effective and reliable patient monitoring solution."
An important contributor in the advancement of modern medical treatment, Nihon Kohden is responsible for the invention of pulse oximetry. Today, with subsidiaries in the USA, Europe and Asia, and distributors in nearly every country in the world, Nihon Kohden is Japan's leading manufacturer, developer and distributor of medical electronic equipment, including: patient monitors, defibrillators, ECGs, EEGs, EP/EMGs, and hematology analyzers.
Masimo Founder and CEO, Joe E. Kiani, stated, "We look forward to continuing our partnership with Nihon Kohden to deliver advanced pulse oximetry solutions to customers in every part of the world. The expansion of our technology license broadens clinical access to 'gold standard' Masimo SET pulse oximetry and reinforces Nihon Kohden's continued commitment to customer satisfaction."
About Nihon Kohden Corporation
Nihon Kohden is Japan's leading manufacturer, developer and distributor of patient monitors, defibrillators, ECGs, EEGs, EP/EMGs, hematology analyzers and other medical electronic equipment, with subsidiaries in the United States, Europe, and Asia, and distributors in nearly every country in the world. Nihon Kohden is a publicly held company listed in the First Section of the Tokyo Stock Exchange. In addition to designing medical equipment for hospital and clinic use, Nihon Kohden actively contributes to the advance of medical technology. For more information, visit the Company's website at www.nihonkohden.com.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET Pulse CO-Oximetry continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumption that this worldwide Masimo SET technology license agreement with Nihon Kohden will serve to increase market acceptance and adoption of the technology, and risk related to our assumption that this technology licensing agreement will serve to substantially increase revenues, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, filed with the Securities and Exchange Commission ("SEC") on October 29, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


ZOLL Enters Multi-year Agreement to Add Masimo Rainbow SET Technology in E Series Defibrillators
Adds the Ability to Diagnose and Treat Carbon Monoxide Poisoning
November 13, 2008—CHELMSFORD, Massachusetts and Irvine, California—ZOLL Medical Corporation, (Nasdaq GS: ZOLL), a manufacturer of resuscitation devices and related software solutions, and Masimo (NASDAQ: MASI), the inventor of Rainbow SET® Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today jointly announced a multi-year technology agreement to integrate Rainbow SET technology into ZOLL E Series® defibrillators. The agreement allows ZOLL to manufacture these defibrillators with this technology that non-invasively and continuously measures carbon monoxide (SpCO) and methemoglobin (SpMet) levels in the blood.
"Masimo Rainbow SET Pulse CO-Oximetry non-invasively delivers the advanced clinical intelligence that today's EMS and fire professionals need to accurately diagnose and treat victims of carbon monoxide poisoning," said Richard A. Packer, Chairman and Chief Executive Officer of ZOLL. "This agreement will allow us to add this important parameter in the future to help rescuers advance their lifesaving efforts."
Carbon monoxide (CO) is a dangerous silent killer that is often very difficult to diagnose. If left untreated, it can have significant morbidity and mortality consequences. Masimo Rainbow SET has the potential to help clinicians improve patient outcomes through more rapid diagnosis of potentially life-threatening conditions—including carbon monoxide poisoning and methemoglobinemia—allowing them to make better and earlier treatment decisions.
Joe E. Kiani, Founder and CEO of Masimo, said, "We're pleased that ZOLL joins us in recognizing the important contribution the cutting-edge technology can make in saving lives."
Masimo Rainbow SET Pulse CO-Oximetry is the first-and-only technology platform capable of noninvasively and continuously measuring total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and pleth variability index (PVI®), in addition to oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). Masimo Rainbow SET—featuring the accuracy and reliability of Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry—significantly expands traditional noninvasive patient monitoring capabilities to quickly and easily measure, track and monitor blood constituents that previously required invasive procedures.
About ZOLL Medical Corporation
ZOLL Medical Corporation is committed to developing technologies that help advance the practice of resuscitation. With products for pacing, defibrillation, circulation, ventilation, and fluid resuscitation, ZOLL provides a comprehensive set of technologies, including Real CPR Help® and See-Thru CPR®, that help clinicians, EMS professionals, and lay rescuers resuscitate sudden cardiac arrest or trauma victims. ZOLL also designs and markets software that automates the documentation and management of both clinical and non-clinical information.
ZOLL markets and sells its products in more than 140 countries. The Company has direct operations, distributor networks, and business partners throughout the U.S., Canada, Latin America, Europe, the Middle East and Africa, Asia, and Australia. 2008 marks the 25th anniversary of ZOLL's resuscitation product development. For more information, visit www.zoll.com.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Certain statements contained in this press release, including statements regarding the future business of the Company, and other statements contained herein regarding matters that are not historical facts, are "forward-looking" statements (as defined in the Private Securities Litigation Reform Act of 1995). Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Factors that could cause actual results to differ materially from those expressed or implied by such forward-looking statements include, but are not limited to, those factors discussed in the section entitled "Risk Factors" in the Company's Quarterly Report on Form 10-Q filed with the SEC on August 8, 2008. You should not place undue reliance on the forward-looking statements in this press release, and the Company disavows any obligation to update or supplement those statements in the event of any changes in the facts, circumstances, or expectations that underlie those statements.
Copyright © 2008 ZOLL Medical Corporation. All rights reserved. 269 Mill Road, Chelmsford, MA 01824-4105. ZOLL, E Series, Real CPR Help, and See-Thru CPR are registered trademarks of ZOLL Medical Corporation. All product names are the property of their respective owners.
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
INVESTOR CONTACT:
A. Ernest Whiton
Chief Financial Officer
ZOLL Medical Corporation
+1 (978) 421-9655
MEDIA CONTACTS:
Diane Egan
ZOLL Medical Corporation
+1 (978) 421-9637
degan@zoll.com
Dana Banks
Masimo Corporation
949-297-7348


Masimo to Present at Lazard Capital Markets 5th Annual Healthcare Conference
IRVINE, Calif., November 6, 2008 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that its management is scheduled to present at the Lazard Capital Markets 5th Annual Healthcare Conference at The St. Regis New York in New York, NY on Wednesday, November 19, 2008, at 9:30 a.m. ET. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (Nasdaq: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and (PVI®), in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at http://www.masimo.com.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Launches First Non-adhesive Pulse Oximetry Sensor Designed For Extremely Low Birth Weight Babies
Unique design, size, and material expected to improve safety and care
Irvine, California – November 5, 2008 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, announced a new SofTouch™ disposable pulse oximetry sensor developed specifically for the fragile skin and size of extremely low birth weight (ELBW) infants (
Barbara Haley, CRTT, NICU Clinical Coordinator, Respiratory Care Services at California Pacific Medical Center in San Francisco, CA, stated; "We love the LNCS SofTouch NeoPt-500 for the improvement in care it facilitates. It is smaller than other neonatal sensors—making it a near-perfect fit even for our smallest preemies—and it is much softer on the skin to maintain skin integrity. We've also found that the NeoPt-500 makes nurses more efficient because the wrap is so much easier to apply and secure."
LNCS SofTouch NeoPt-500 is the first non-adhesive pulse oximetry sensor made specifically for the needs of newborns weighing down to 500 grams or less. At just 20 mm in size, the NeoPt-500 has dimensions that are smaller than typical neonatal sensors. This means that the distance between the emitter and detector on the NeoPt-500 is much smaller—enabling the sensor to fit better and more securely on the tiniest hand or foot.
Mitchell Goldstein, M.D., Associate Professor, Pediatrics at Loma Linda University Children's Hospital in Loma Linda, CA, stated; "The new LNCS® SofTouch NeoPt-500 sensor solves the problems associated with typical pulse oximetry sensors in ELBW infants. ELBW neonates are much more susceptible to skin burn injury and full thickness ulcer formations caused by improper sensor design and placement. Skin injury compromises the body's most basic protection mechanism and, in this most vulnerable population, the problems and risks associated with sensors not designed or intended for ELBW newborns have much greater consequences. Masimo is a unique company that not only understands this, but designs and develops clinically-relevant solutions to overcome these challenges."
Masimo SofTouch sensors are designed for single-patient use whenever skin sensitivity is a concern. SofTouch sensors incorporate a hook and loop wrap—allowing the sensor to be quickly and securely applied on wet and slippery sites, and easily repositioned if necessary. Newborn skin is further protected by soft foam material that surrounds the sensor attachment for maximum comfort, and the NeoPt-500 contains no adhesive materials exposed to skin contact areas. This prevents epidermal stripping and skin trauma typically caused by applying and repositioning an adhesive sensor.
Joe E. Kiani, Founder and CEO of Masimo, stated; "The new LNCS SofTouch NeoPt-500 sensor underscores our core philosophy of 'doing what is best for patient care.' We believe that every patient deserves appropriate monitoring solutions. That's why Masimo provides over 100 different sensors, each uniquely designed to deliver the performance and measurement clinicians want – including 15 different sensor options to address the challenging needs and specific requirements of neonatal patients."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumption that the addition of the LNCS® NeoPt-500 SofTouch™® sensor will outperform other neonatal pulse oximetry sensors and will serve to increase clinical accuracy and efficiency and the adoption of Masimo technology, or serve to substantially increase sensor sales, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, filed with the Securities and Exchange Commission ("SEC") on October 29, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended September 27, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports Third Quarter 2008 Financial Results
Record results mark 21st consecutive quarter of revenue growth
Q3 2008 Highlights:
- Product revenues increased 29% to a record $66.0 million
- Direct (Non-OEM) Business revenues increased by 48%
- Shipped 30,700 new pulse oximeters and Pulse CO-Oximeters
Irvine, California, October 27, 2008 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, today announced its financial results for the 2008 third fiscal quarter.
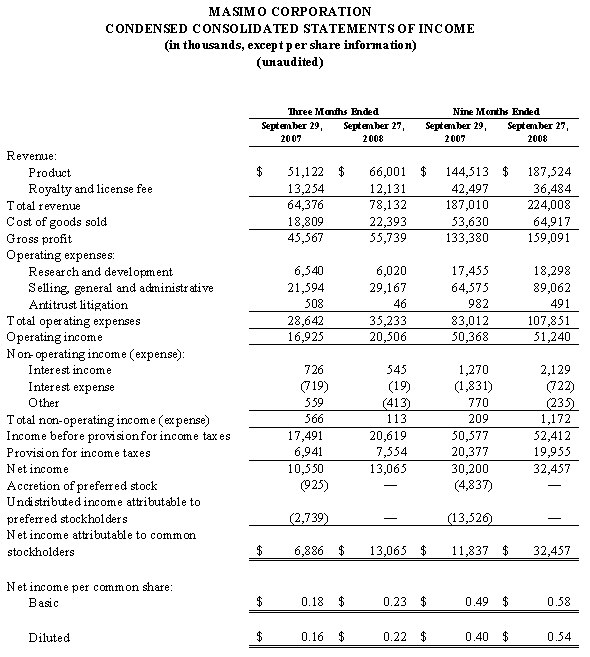
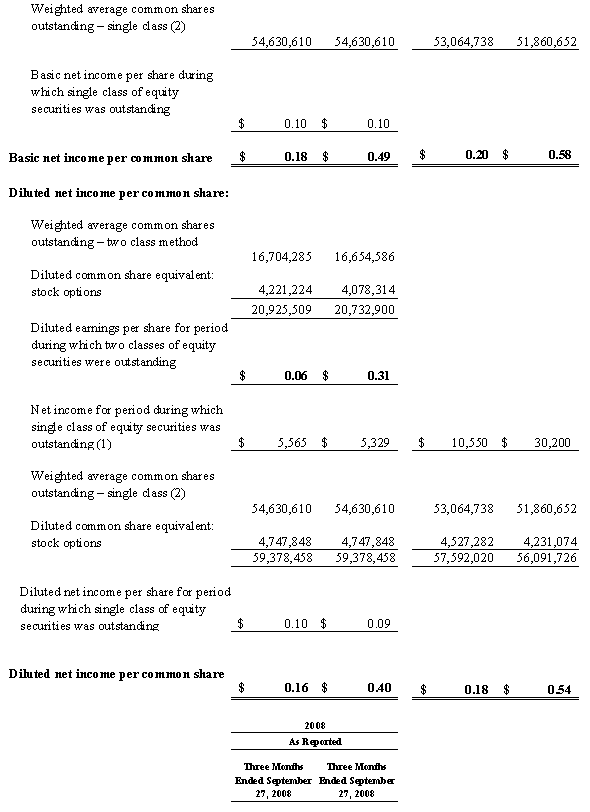
For the third quarter of 2008, Masimo reported product revenues of $66.0 million representing a 29% increase over $51.1 million for the third quarter of 2007. Including royalty revenues, Masimo reported total 2008 third quarter revenues of $78.1 million compared to $64.4 million for the third quarter of 2007. Net income for the 2008 third quarter was $13.1 million or $0.22 per common share compared to $6.9 million or $0.16 per common share for the third quarter of 2007.
During the third quarter of 2008, Masimo also reported that it shipped 30,700 Masimo SET and Masimo Rainbow SET oximetry units, excluding handheld units, compared to 30,800 units in the same prior year quarter. For the first nine months of 2008, Masimo has shipped 88,400 new units representing an annualized increase of 25% over the estimated 470,000 Masimo units in the market as of December 2007 and has now increased its total worldwide net installed base to 540,000 units.
In the third quarter of 2008, revenues from Masimo Rainbow SET products increased by 85% from the same prior year quarter to $3.0 million. For the first nine months of 2008, revenues from Masimo Rainbow SET products were $8.7 million, an increase of 71% compared to the first nine months of 2007.
Joe E. Kiani, Chairman and Chief Executive Officer of Masimo, said, "We are happy with the continued strength in our core pulse oximetry business and growth in Rainbow SET product revenues. Our business model, which is based on innovation and recurring revenue from our installed base, is showing resilience in a difficult economic environment."
As of September 27, 2008, cash and cash equivalents totaled $122.6 million, an increase from December 29, 2007 of $96.7 million. As previously announced, during the 2008 first fiscal quarter, Masimo repaid in full a $26.7 million debt obligation, the majority of which was established in early fiscal 2007.
Financial Guidance
Based on the information available as of October 27, 2008, relating to the results of the first nine months of fiscal year 2008, Masimo now expects its total fiscal year 2008 product revenues to be approximately $256 million and total revenues, including royalties, to be approximately $303 million. These figures are up from the previously released guidance of $253 million and $300 million, respectively. Masimo also now expects earnings per common share for fiscal year 2008 to be approximately $0.71 per share, up from the previously released guidance of $0.64 per share.
Conference Call
Masimo will hold a conference call today at 2:00 p.m. PT (5:00 p.m. ET) to discuss the results. The dial-in numbers are (800) 399-7902 for domestic callers and (706) 634-0949 for international callers. The reservation number for both dial-in numbers is 68032269. A live webcast of the conference call will be available online from the "Investor Relations" page of the Company's corporate website at www.masimo.com. After the live webcast, the call will remain available on Masimo's website through November 27, 2008. In addition, a telephonic replay of the call will be available until November 9, 2008. The replay dial-in numbers are (800) 642-1687 for domestic callers and (706) 645-9291 for international callers. Please use reservation code 68032269. Our financial guidance will be limited to the statements made in this press release.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements. All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations, prospects and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and expectations for total revenues, including royalties, product revenues and GAAP earnings per share for the full fiscal year 2008. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our reliance on Masimo SET and related products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; the failure to continue developing innovative products and technologies; the lack of acceptance of any new products and technologies of ours; obtaining regulatory approval of our current and future products and technologies, including the recently announced total hemoglobin measurement; the loss of our customers the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the U.S. credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended June 28, 2008 filed with the Securities and Exchange Commission on August 5, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended June 28, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.





New Clinical Studies Presented at the American Society of Anesthesia (ASA) Annual Meeting Show Masimo Technologies Provide Advanced Clinical Intelligence
Masimo's Commercial Exhibit Draws More Than 2,500 Conference Attendees
Irvine, California – October 23, 2008 – Masimo, the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that 13 new independent and objective clinical studies presented at the American Society of Anesthesiology (ASA) Annual Meeting in Orlando, Fla., October 19-21, 2008, found Masimo SET® and Masimo Rainbow SET® technologies to be crucial in providing clinicians with an early indication of their patients' deteriorating health status in numerous clinical settings, which may allow earlier intervention and prevention of adverse clinical outcomes.
In addition, Masimo's commercial exhibit attracted over 2,500 anesthesiologists from the U.S. and around the world. Anesthesiologists visiting the commercial exhibit marveled at Masimo noninvasive hemoglobin (SpHb™), the first-and-only technology capable of continuously monitoring hemoglobin status. Continuous monitoring of anemic status with Masimo SpHb may allow clinicians to make earlier and better treatment decisions, such as detecting blood loss earlier to initiate more timely transfusions in some patients and avoiding unnecessary blood transfusions in others.
Select Clinical Study Highlights
Continuous Pulse Ox Impacts Early Detection of Physiological Abnormalities in Post-Surgical Patients1, a clinical evaluation led by Dr. George Blike at Dartmouth-Hitchcock Medical Center in Lebanon, New Hampshire, compared clinical data, patient outcomes and nursing satisfaction before and after implementation of Masimo Patient SafetyNet™—a remote monitoring and wireless clinician notification system—on a 36-bed post-surgical care unit. Findings during the three-month evaluation—covering 2,587 total patient days—showed an 80 percent decrease in distress codes and rescue activations, and a 50 percent decrease in ICU transfers for Masimo Patient SafetyNet system-monitored patients. Researchers concluded that, "the system supported the early identification of patients with sedation or analgesia-induced respiratory depression," as well as the "early recognition of other patterns of deterioration, including: poor heart rate control, acute bradycardia needing atropine, new onset A-fib, unrecognized obstructive patterns of respiration like sleep apnea, and pulmonary complications such as fat emboli syndrome, pulmonary embolus and edema." In addition, researchers also noted that the system rated high for up-time at 99.9995% and nursing satisfaction, as identified by 'desire to keep vs. remove the system', at 5.5 on a 6 point scale.
Do Pulse CO-Oximeter Measures of SpMet and SpO2 Correlate with Blood Gas CO-Oximetry in Neonates?2, a clinical study led by Dr. Mitchell R. Goldstein at Loma Linda Children's Hospital in Loma Linda, California, evaluated whether methemoglobin saturation (SpMet™) could be successfully measured in neonates without compromise in oxygen saturation (SpO2) accuracy. Researchers compared noninvasive SpMet and SpO2 measurements obtained from neonates using the Masimo Radical-7 Pulse CO-Oximeter and Rainbow R25-L disposable sensor with MetHb and SaO2 measurements obtained from blood gas analysis using a laboratory CO-oximeter and found that the accuracy of SpMet (with a Bias of 0.17, Standard Deviation of 0.92, and Average Root Mean Square of 0.93) and SpO2 (with a Bias of 1.4, Standard Deviation of 2.46, and Average Root Mean Square of 2.86) was maintained and correlated with blood gas measurements. Researchers concluded that Masimo's multi-wavelength Pulse CO-Oximeter "can simultaneously measure SpMet and SpO2 in neonates," and that "continuous monitoring of MetHb allows better assessment of toxicity and helps identify the need for ongoing treatment."
Ability of Pleth Variability Index to Non-Invasively Predict the Hemodynamic Effects of PEEP3—led by Dr. Olivier Desebbe at Louis Pradel Hospital in Lyon-Bron, France, studied the ability of PVI to predict the effects of positive end-expiratory pressure (PEEP) on cardiac index (CI) in 21 mechanically-ventilated patients following cardiac surgery. The clinical team recorded mean arterial pressure (MAP), central venous pressure (CVP), cardiac index (measured using pulmonary artery catheter), and PVI at three successive tidal volume settings (6, 8, and 10 ml/kg) under zero end-expiratory pressure and following adjunction of a PEEP, and found that successive zero end-expiratory pressure induced significant changes in PVI, but not CVP or MAP. Findings also showed that "PVI was able to predict the hemodynamic effect of PEEP with 73% sensibility and 80% specificity." Researchers concluded that PVI could allow clinicians to "optimize fluid loading noninvasively before adding PEEP for pulmonary reasons."
Pulse Oximeter Perfusion Index as a Predictor for the Effect of llio-Inguinal Block4, a prospective clinical study led by Dr. AKI Uemura at the Tsukuba University Hospital in Ibaraki, Japan, examined whether changes in Perfusion Index (PI) reflect the effect of llio-inguinal block in 18 children (mean age 32 months) during inguinal herniorrhaphy. Patients receiving llio-inguinal blocks were divided into two groups according to the concentration of Ropivacaine received (0.25% or 0.5%), and monitored using electrocardiography, noninvasive blood pressure, and two Masimo SET Radical pulse oximeters placed on both the left and right side limb. The clinical team recorded PI, blood pressure, heart rate, end-tidal CO2, end-tidal Sevo%, and respiratory rate for all patients and found that PI on the block side was significantly elevated when compared to the non-block side. Researchers concluded that "PI value is a useful, objective, and noninvasive method to evaluate the effect of llio-inguinal block in pediatric patients."
In addition, there were nine other clinical studies5-13 presented validating the accuracy, reliability, and clinical value of Masimo SET Pulse Oximetry and Rainbow SET Pulse CO-Oximetry—the first-and-only technology platform to noninvasively measure blood constituents and fluid responsiveness that previously required invasive procedures, including: noninvasive & continuous total hemoglobin (SpHb), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®) and Pleth Variability Index (PVI), in addition to the measure-through-motion-and-low-perfusion performance of Masimo SET Oxygen Saturation (SpO2), Pulse Rate (PR) and Perfusion Index (PI).
Michael O'Reilly, MD, Masimo Executive Vice President of Medical Affairs, stated; "The 13 new studies presented at ASA this year add to the growing body of evidence showing that the use of Masimo SET pulse oximetry and Masimo Rainbow SET Pulse CO-Oximetry improves patient safety and outcomes."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that the applications of Masimo Rainbow SET technologies described in the foregoing clinical studies will deliver a sufficient level of clinical improvement over alternative measurement capabilities to allow for rapid adoption of the technology, and risks related to our assumptions regarding the repeatability of clinical results at other hospitals and healthcare settings, and risks related to our assumptions regarding timing or commercial availability of SpHb, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended June 28, 2008, filed with the Securities and Exchange Commission ("SEC") on August 5, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended June 28, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
1 Continuous Pulse Ox Impacts Early Detection of Physiological Abnormalities in Post-Surgical Patients. George Blike, M.D., Jean Avery, R.N., Melanie Mastanduno, R.N., Klaus Christoffersen, Ph.D., Susan McGrath, Ph.D. Quality & Patient Safety, Dartmouth-Hitchcock Medical Center, Lebanon, NH.
2 Do Pulse CO-Oximeter Measures of SpMet and SpO2 Correlate with Blood Gas CO-Oximetry in Neonates. Mitchell R. Goldstein, M.D., Daniel Saesim, M.D., Mark Macknet, M.D., Martin Allard, M.D., Ricardo Peverini, M.D. Neonatal Medicine, Loma Linda University Children's Hospital, Loma Linda, California.
3 Ability of Pleth Variability Index to Non-Invasively Predict the Hemodynamic Effects of PEEP. Olivier Desebbe, M.D., Cecile Boucau, R.D., Pascal Rosamel, M.D., Jean-Jacques Lehot, M.D., Ph.D., Maxime Cannesson, M.D. Department of Anesthesiology, Louis Pradel Hospital, Lyon-Bron, France.
4 Pulse Oximeter Perfusion Index as a Predictor for the Effect of llio-Inguinal Block. Aki Uemura, M.D., Ph.D., Masahiro Yagihara, M.D., Masayuki Miyabe, M.D., Ph.D. Anestheiology, Tsukuba University, Ibaraki, Japan.
5 Ability of PVI to detect preload changes in ortho liver transplant. Christopher Wray, M.D., Jack Buckley, M.D., Derek Kwan, B.S., Tayeba Maktabi, Aman Mahajan, M.D., Ph.D. Anesthesiology, David Geffen School of Medicine, UCLA, Los Angeles, California.
6 PVI A non-invasive device for fluid responsiveness. Olivier Desebbe, M.D., Cecile Boucau, R.D., Pascal Rosamel, M.D., Jean-Jacques Lehot, M.D., Ph.D., Maxime Cannesson, M.D. Department of Anesthesiology, Louis Pradel Hospital, Lyon-Bron, France.
7 Impact of PEEP on PI and PVI. Nitin K. Shah, M.D., Darin V. Allred, M.D., Laverne Estanol, M.S., Fine Brian, B.S., Ghandi Vipal, B.S. Anesthesiology, Long Beach VAHS, Long Beach, California.
8 Impact of lower extreminity nerve blockade on PI and PVI. Darin V. Allred, M.D., Nitin K. Shah, M.D., Laverne Estanol, M.S. Anesthesiology, University of California Irvine, Orange, California.
9 Perfusion Index via finger and toe. Hiroyuki Sumikura, M.D., Ph.D., Yayoi Ohashi, M.D., Ph.D., Yasuyuki Suzuki, M.D., Youichi Kondo, M.D., Hirokazu Sakai, M.D. Obstetric Anesthesia, National Center for Child Health and Development, Tokyo, Japan.
10 Effect of Servoflurane on peripherial PI. Anne Laffargue, M.D., Bruno Marciniak, M.D., Anne Hebrard, M.D., Caroline Petyt, M.D., Renee Krivosic-Horber, M.D. Pole de'Anethesie Reanimation, Jeanne de Flandre, CHRU, Lille, France.
11 Prolocaine induced Methemoglobinemia. Peter Soeding, M.D., Matthias Deppe, Hartmut Gehring, M.D., Ph.D. Anesthesiology, University of Luebeck, Lubeck, Schleswig-Holstein, Germany.
12 Second hand smoke in children. Branden E. Yee, B.A., Iqbal M. Ahmed, M.D., Raghu Idupuganti, D.O., Douglas Brugge, Ph.D., M.S., Roman Schumann, M.D. Anesthesia, Tufts Medical Center, Boston, MA.
13 Estimation of respiration dependent PaO2 Oscillations. Marc Bodenstein, M.D., Stephan Boehme, John Graybeal, M.D., Hemei Wang, Ph.D., Klaus Markstaller, M.D., Ph.D. Department of Anesthesiology, Johannes Gutenber-University, Mainz, Rhineland-Palatinate, Germany.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Five New Clinical Studies Show Value of Masimo PVI & PI
Data to be presented at American Society of Anesthesiologists Annual Meeting as Masimo showcases world's first-and-only noninvasive and continuous hemoglobin (SpHb) and Oxygen Content (SpOC) monitor with live booth demonstrations
Irvine, California (October 17, 2008)--Masimo, the inventor of Pulse CO-Oximetry™ and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, announced today that five new independent and objective studies demonstrating Masimo PVI to be highly predictive of patient fluid status will be presented at the American Society of Anesthesiology (ASA) Annual Meeting in Orlando, Fla., October 19-21, 2008.
Fluid administration is critical to optimizing patient status and enabling end organ preservation, but traditional methods to guide fluid administration are invasive and often fail to predict fluid responsiveness. PVI has been shown to predict fluid responsiveness in mechanically ventilated patients under general anesthesia during surgery, and may help clinicians optimize fluid administration and improve patient outcomes.2-4
Also at ASA for the first time, Masimo will be showcasing live demonstrations of the first-ever noninvasive and continuous hemoglobin (SpHb) and Oxygen Content (SpOC) monitor. SpHb provides instantaneous hemoglobin measurements that may facilitate faster, easier, safer, and better clinical decisions by allowing clinicians to more quickly detect chronic or acute anemia, identify occult bleeding earlier, and more effectively manage blood transfusions. While oxygen saturation (SpO2) and hemoglobin are considered critical parameters for patient management, neither parameter by itself can indicate the actual amount of oxygen in the blood. However, now with Masimo Rainbow SET Pulse CO-Oximetry, SpHb and SpO2 can be used together to provide clinicians with the first-and-only technology for real-time and noninvasive oxygen content (SpOC™) monitoring, providing a more complete picture of oxygenation status and potentially allowing earlier indication of when a patient crosses the threshold into a critical oxygen deficit.
The following studies will be presented at the ASA Annual Meeting:
—Impact of PEEP on Perfusion Index and Plethysmographic Variability Index1
A1068, Presented: October 20, 2008, 2:00-4:30 p.m., Room Hall E2-Area M
—Ability of Pleth Variability Index to Non-Invasively Predict the Hemodynamic Effects of PEEP2
A1608, Presented: October 22, 2008, 8:00-9:30 a.m., Room 230C
—Ability of Pleth Variability Index to Detect Preload Changes in Orthotopic Liver Transplant Patients3
A1605, Presented: October 22, 2008, 8:00-9:30 a.m., Room 230C
—Pleth Variability Index: A Noninvasive Device for Fluid Responsiveness Assessment during Anesthesia4
A1604, Presented: October 22, 2008, 8:00-9:30 a.m., Room 230C
—Impact of Lower Extremity Nerve Blockage on Oximeter Perfusion Index & Pleth Variability Index5
A1603, Presented: October 22, 2008, 8:00-9:30 a.m., Room 230C
Michael O'Reilly, MD, EVP of Medical Affairs at Masimo, stated; "These studies add to the evidence showing that PVI can provide clinicians with an effective and efficient noninvasive method of continuously measuring their patient's fluid volume. This should enable more accurate fluid administration decisions—allowing clinicians to add a level of certainty and immediacy toward managing intravascular fluid volumes and cardiac output both inside and outside of the operating room."
PVI is available as part of Masimo Rainbow SET® Pulse CO-Oximetry™—the first-and-only technology platform to noninvasively measure blood constituents and fluid responsiveness that previously required invasive procedures, including: noninvasive & continuous total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®) and Pleth Variability Index (PVI), in addition to the measure-through-motion-and-low-perfusion performance of Masimo SET® Oxygen Saturation (SpO2), Pulse Rate (PR) and Perfusion Index (PI).
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHbTM), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVITM, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo PVI and total hemoglobin (SpHbTM) will deliver a sufficient level of clinical improvement over alternative fluid assessment and hemoglobin measurement capabilities to allow for rapid adoption of the technology, risks related to our assumptions regarding the repeatability of clinical results, and risks related to our assumptions regarding timing or commercial availability of SpHb, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended June 28, 2008, filed with the Securities and Exchange Commission ("SEC") on August 5, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended June 28, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
1 Impact of PEEP on Perfusion Index and Plethysmographic Variability Index. Nitin K. Shah, M.D., Darin V. Allred, M.D., Laverne Estanol, M.S., Brian Fine, B.S., Gandhi Vipal, B.S. Anesthesiology, Long Beach VAHS, Long Beach, California.
2 Ability of Pleth Variability Index to Non-Invasively Predict the Hemodynamic Effects of PEEP. Olivier Desebbe, M.D., Cecile Boucau, R.D., Pascal Rosamel, M.D., Jean-Jacques Lehot, M.D., Ph.D., Maxime Cannesson, M.D. Department of Anesthesiology, Louis Pradel Hospital, Lyon-Bron, France.
3 Ability of Pleth Variability Index to Detect Preload Changes in Orthotopic Liver Transplant Patients. Christopher Wray, M.D., Jack Buckley, M.D., Derek Kwan, B.S., Tayeba Maktabi, Aman Mahajan, M.D., Ph.D. Anestheiology, UCLA David Geffen School of Medicine, Los Angeles, California.
4 Pleth Variability Index: A Noninvasive Device for Fluid Responsiveness Assessment during Anesthesia. Olivier Desebbe, M.D., Bertrand Delannoy, R.A., Jean-Jacques Lehot, M.D., Ph.D., Olivier Bastien, M.D., Ph.D., Maxime Cannesson, M.D. Department of Anesthesiology, Louis Pradel Hospital, Lyon-Bron, France.
5 Impact of Lower Extremity Nerve Blockage on Oximeter Perfusion Index & Pleth Variability Index. Darin V. Allred, M.D., Nitin K. Shah, M.D., Laverne Estanol, M.S. Anesthesiology, University of California at Irvine, Orange, California.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report Third Quarter 2008 Financial Results on October 27, 2008
Conference call and webcast to begin after markets close at 2:00 p.m. PT (5:00 p.m. ET)
IRVINE, Calif., October 15, 2008—Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced it will release third quarter financial results for the period ended September 27, 2008 after the market closes on Monday, October 27, 2008.
A conference call to review the results will begin at 2:00 p.m. PT (5:00 p.m. ET) and will be hosted by Joe E. Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the Company's corporate website at www.masimo.com. The dial-in numbers are (800) 399-7902 for domestic callers and +1 (706) 634-0949 for international callers. The reservation number for both dial-in numbers is 68032269. After the live webcast, the call will remain available on Masimo's website through November 27, 2008. In addition, a telephonic replay of the call will be available until November 9, 2008. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 68032269.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
949-297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Announces $1 Million Performance Guarantee
Launches 'Fact vs. Fiction' website.
Irvine, California, October 2, 2008 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced a $1 million performance guarantee and unveiled a new website aimed at separating fact from fiction. The 'Fact vs. Fiction' website, www.nellcorfiction.com, will openly address and dissect erroneous and misleading information circulated by Nellcor, a division of Covidien.
The superior performance and clinical benefits of Masimo SET pulse oximetry technology are proven everyday at thousands of hospitals, and reinforced by more than 100 independent and objective studies demonstrating that Masimo provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion.
Masimo is so confident in its ability to provide clinicians with the best performing pulse oximetry technology, the company is backing up its technology with a $1 million performance guarantee for hospitals planning to upgrade their pulse oximetry technology hospital-wide. If Masimo SET does not outperform Nellcor in an objective side-by-side clinical evaluation, Masimo will pay the hospital up to $1 million to acquire Nellcor's pulse oximetry. Additional performance guarantee details can be found online at www.masimo.com.
Joe E. Kiani, Founder and CEO of Masimo, stated; "The Fact vs. Fiction website was developed in response to reports about Nellcor's constant and aggressive dissemination of false information to clinicians. The website exposes Nellcor fictions and delivers facts backed by proof. Our dedication and commitment to 'improving patient outcomes and reducing cost of care by taking noninvasive monitoring to new sites and applications' means that we not only owe it to care providers to innovate breakthroughs in noninvasive monitoring, but we must also do our part to educate, and sometimes even expose obfuscations and falsehoods."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET is superior to all other commercially-available pulse oximetry technologies, and risks related to our assumption that truths asserted in the 'Fact vs. Fiction' website (www.nellcorfiction.com) will serve to substantially increase awareness of Nellcor fictions and/or Masimo's superiority, as well as other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended June 28, 2008, filed with the Securities and Exchange Commission ("SEC") on August 5, 2008, which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended June 28, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks
Manager, Public Relations
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Initiates Limited Release of First-ever Noninvasive and Continuous Hemoglobin Monitors
Masimo Receives FDA Clearance for Noninvasive Total Hemoglobin Adhesive Sensors
Irvine, California – September 15, 2008 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced limited release of its noninvasive and continuous hemoglobin (SpHb™) and oxygen content (SpOC™) monitors, Radical-7 and Rad-87. Masimo also announced the FDA 510(k) clearance of its latest Rainbow family of adhesive sensors capable of noninvasively measuring total hemoglobin and oxygen content, along with oxygen saturation (SpO2), pulse rate, pleth variability index (PVI), and methemoglobin (SpMet®), for use with Masimo Rainbow SET-enabled devices.
The limited release of Masimo Radical-7 and Rad-87 Pulse CO-Oximeters with noninvasive and continuous total hemoglobin technology will allow select hospitals around the world to be the first to pioneer a breakthrough technology that may make it safer, faster, and easier for healthcare professionals to measure hemoglobin—without removing a drop of blood. Hospitals purchasing noninvasive hemoglobin devices and selected for the limited product release will work closely with Masimo to implement the technology in their facilities and provide data on its impact on patient care.
"Current methods for measuring total hemoglobin are invasive and only offer delayed and intermittent results," stated Joe E. Kiani, Masimo Chairman and CEO. "We look forward to working closely with the first customers of our noninvasive hemoglobin monitoring technology to enable earlier and better clinical decisions, improve patient safety, and decrease costs."
Today, hemoglobin is one of the most commonly-performed clinical laboratory tests with more than 400 million performed annually in the U.S. alone. A low hemoglobin level is called anemia, a pervasive blood disorder that according to the World Health Organization (WHO) affects two billion people worldwide and causes one million deaths a year. Anemia can be chronic or acute. Chronic anemia is characterized by consistently low hemoglobin levels that can be the result of a diet deficiency, or illness such as cancer. Acute anemia is characterized by a sudden drop in hemoglobin levels that can result from internal or external bleeding due to surgery or trauma.
Masimo noninvasive total hemoglobin (SpHb™) and oxygen content (SpOC™) are part of Masimo Rainbow SET Pulse CO-Oximetry, the first and only technology platform to noninvasively measure blood constituents and fluid responsiveness that previously required invasive procedures. In addition to hemoglobin and oxygen content, Rainbow measurements include: carboxyhemoglobin (SpCO®), methemoglobin (SpMet®) and Pleth Variability Index (PVI)—in addition to the measure-through-motion-and-low-perfusion performance of Masimo SET Oxygen Saturation (SpO2), Pulse Rate (PR) and Perfusion Index (PI).
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that Masimo noninvasive and continuous total hemoglobin technology will provide faster, easier and safer means for measuring total hemoglobin and will lead to additional device sales, and risks related to our assumption that noninvasive total hemoglobin will be available in the first quarter of 2009, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended June 28, 2008, filed with the Securities and Exchange Commission on August 5, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended June 28, 2008, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation


Masimo Announces Desaturation Index 3D Alarm as Standard Feature in All Radical-7 Pulse CO-Oximetry Devices
Irvine, California – September 10, 2008 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced that all customers purchasing the Masimo Radical-7, the company's flagship product, will now receive Desat Index 3D Alarm as a standard feature.
Conventional pulse oximeters typically alarm based on large and isolated drops in oxygen saturation values, known as a "desaturation". However, published research has shown that a cycle of moderate desaturations has been shown to precede respiratory failure in hospitalized patients.1, 2 Masimo Desat Index 3D Alarm enables advanced notification of these conditions, based on clinician-specified severity and number of desaturations occurring in a specified period of time.
Michael O'Reilly, MD, Masimo Executive Vice President of Medical Affairs, stated; "When combined with the proven ability of Masimo Signal Extraction Technology (SET) to dramatically reduce false desaturation alarms and improve the detection of true desaturations, Masimo Desat Index 3D Alarm advances patient care by allowing earlier intervention to prevent adverse respiratory events."
Joe Kiani, Chairman and CEO of Masimo, stated; "Masimo has been selling Desat Index 3D Alarm as an option for almost three years. In light of our soon-to-be released breakthrough innovation, noninvasive and continuous hemoglobin monitoring technology, we are pleased to now offer Desat Index 3D Alarm as a standard feature."
1 Continuous Pulse Oximeter Monitoring for Inapparent Hypoxemia after Long Bone Fractures. Wong MW, Tsui HF, Yung SH, Chan KM, Cheng JC. Journal of Trauma-Injury Infection & Critical Care. 56(2):356-362, February 2004.
2 Occurance and Mechanisms of Sudden Oxygen Desaturation in Infants Who Sleep Face Down. Patel AL, Paluszynska D, Harris KA, Thach BT.Pediatrics. 2003 Apr;111(4 Pt 1):e328-32.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that the addition of Desat Index 3D Alarms as a standard feature on the Masimo Radical-7 will lead to additional device sales , as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended June 28, 2008, filed with the Securities and Exchange Commission on August 5, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended June 28, 2008, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Announces Dismissal of Litigation Brought by Shaklee and NIR Diagnostics
Irvine, California, August 27, 2008 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced that it has settled pending litigation brought by Shaklee Corporation and NIR Diagnostics, Inc. against Masimo. Pursuant to the settlement, Shaklee and NIR dismissed all outstanding claims against Masimo in the litigation with prejudice, and Masimo dismissed all outstanding claims against Shaklee and NIR without prejudice. The settlement was reached without Masimo incurring any liability to either Shaklee or NIR.
On July 24, 2007, just prior to Masimo's initial public offering, Shaklee filed a lawsuit against Masimo in the United States District Court for the Central District of California. NIR later joined Shaklee as a plaintiff. Shaklee and NIR alleged that Masimo's pulse oximeters incorporated patented calibration methods licensed by NIR to Shaklee. Shaklee and NIR sought an injunction and damages against Masimo. On August 13, 2008, Masimo and Shaklee filed a joint stipulation dismissing the litigation with the District Court, which the District Court then entered as an order; and, on August 22, 2008, Masimo and NIR filed a joint stipulation dismissing the litigation with the District Court, which the District Court then entered as an order.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at Thomas Weisel Partners Healthcare Conference 2008
IRVINE, Calif., August 26, 2008 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced that it is scheduled to present at the Thomas Weisel Partners Healthcare Conference 2008 at The Four Seasons Boston Hotel in Boston, MA, on Thursday, September 4, 2008, at 9:10 a.m. EST. Joe E. Kiani, Chairman and CEO, will be presenting.
A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (Nasdaq: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports Second Quarter 2008 Financial Results
Record results mark 20th consecutive quarter of revenue growth
Q2 2008 Highlights:
- Product revenues increased 30% to a record $61.9 million
- Direct (Non-OEM) Business revenues increased by 46%
- Shipped 29,000 new pulse oximeters and Pulse CO-Oximeters
Irvine, California, August 4, 2008 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced its financial results for the 2008 second fiscal quarter.
For the second quarter of 2008, Masimo reported product revenues of $61.9 million representing a 30% increase over $47.6 million for the second quarter of 2007. Including royalty revenues, Masimo reported total 2008 second quarter revenues of $74.8 million compared to $63.7 million for the second quarter of 2007. Net income for the 2008 second quarter was $10.6 million or $0.18 per common share compared to $10.6 million or $0.13 per common share for the second quarter of 2007.
During the second quarter of 2008, Masimo also reported that it shipped 29,000 Masimo SET and Masimo Rainbow SET oximetry units, excluding handheld units, compared to 29,700 units in the same prior year quarter. For the first half of 2008, Masimo shipped 57,600 new units representing an annualized increase of 24% over the estimated 471,000 Masimo units in the market as of December 2007 and has now increased its total worldwide net installed base to 515,000 units. In the second quarter of 2008, revenues from Masimo Rainbow SET products increased 40% from the same prior year quarter.
Joe E. Kiani, Chairman and Chief Executive Officer of Masimo, said, "We believe that the benefit to patients, clinicians and hospitals of our life saving Masimo SET and Masimo Rainbow SET technologies continue to create momentum for the adoption of our products. Despite the generally difficult economic environment, we believe our business model continues to resonate with hospitals who demand the best technology for their patients. In addition, after a strong first quarter, we are pleased that sales of our Rainbow SET products have continued to gain momentum and set a new record in the second quarter of 2008."
As of June 28, 2008, cash and cash equivalents totaled $102.9 million—an increase from both December 29, 2007 of $96.7 million and from March 29, 2008 of $86.3 million. As previously announced, during the 2008 first fiscal quarter, Masimo repaid in full a $26.7 million debt obligation, the majority of which was established in early fiscal 2007.
Financial Guidance
Based on the results of the first half of fiscal year 2008, Masimo now expects its total fiscal year 2008 product revenues to be approximately $253 million and total revenues, including royalties, to be approximately $300 million. These figures are up from the previous guidance of $246 million and $292 million, respectively. Masimo also now expects earnings per common share to be approximately $0.64 per share, up from the prior guidance of $0.52 per share.
Conference Call
Masimo will hold a conference call today at 2:00 p.m. PT (5:00 p.m. ET) to discuss the results. The dial-in numbers are (866) 356-3095 for domestic callers and (617) 597-5391 for international callers. The reservation number for both dial-in numbers is 90085495. A live webcast of the conference call will be available online from the "Investor Relations" page of the Company's corporate website at www.masimo.com.
After the live webcast, the call will remain available on Masimo's website through September 4, 2008. In addition, a telephonic replay of the call will be available until August 18, 2008. The replay dial-in numbers are (888) 286-8010 for domestic callers and (617) 801-6888 for international callers. Please use reservation code 86583404.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements. All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations, prospects and business generally; the market acceptance of our technologies and products; the value of measuring new parameters; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and expectations for total revenues, product revenues, GAAP earnings per share, non-GAAP pro forma earnings per share and stock based compensation expenses for the full fiscal year 2008. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our reliance on Masimo SET and related products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; the failure to continue developing innovative products and technologies; the lack of acceptance of any new products and technologies of ours; obtaining regulatory approval of our current and future products and technologies, including the recently announced total hemoglobin measurement; the loss of our customers the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our Quarterly Report on Form 10-Q for the fiscal quarter ended March 29, 2008 filed with the Securities and Exchange Commission on May 1, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our Quarterly Report on Form 10-Q for the fiscal quarter ended March 29, 2008, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
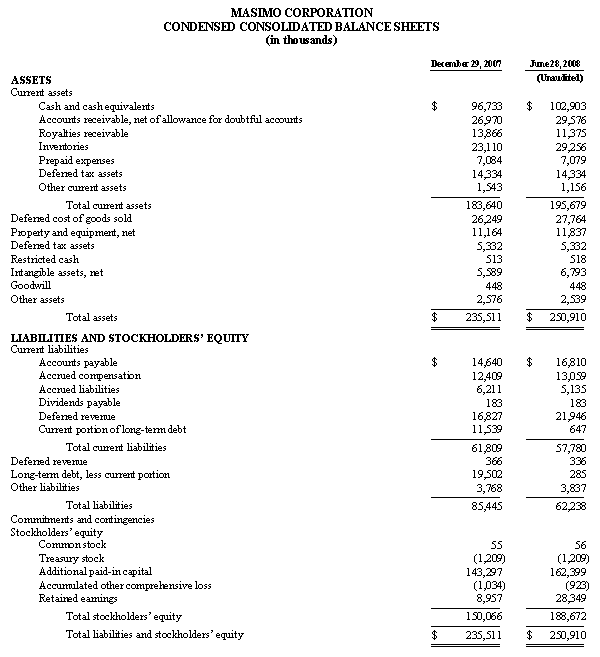
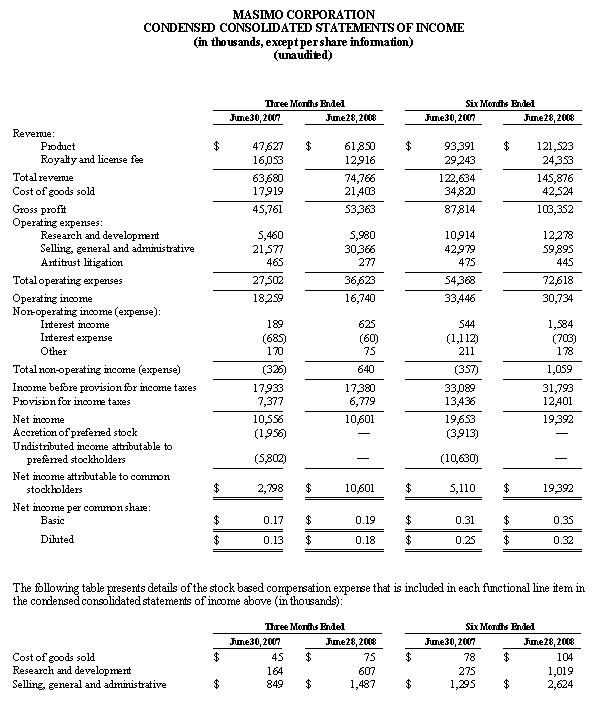


Aurora Health Care Completes System-Wide Conversion to Masimo SET Pulse Oximetry Technology
Masimo provides Aurora with technology and sensor standardization across its facilities—creating efficiencies and cost savings
Irvine, California—July 31, 2008 – Masimo, the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion Pulse Oximetry, announced that Aurora Health Care has completed a system-wide conversion to Masimo SET pulse oximetry technology. The integrated health care provider with 13 hospitals and more than 100 clinics performed an independent evaluation of pulse oximetry technologies and chose Masimo over all other available technologies to unify patient monitoring capabilities across their entire network.
"The decision to convert all of our hospital and surgery facilities to Masimo SET pulse oximetry originally grew out of clinical preference in the Post Anesthesia Care Unit (PACU) and Neonatal Intensive Care Unit (NICU) because of Masimo's superior performance during the most challenging of patient conditions," stated Alan Gresch, Corporate Manager of Clinical Engineering, Aurora Health Care. "Performance preference soon gave way to clinical efficiencies and cost savings created by standardization to the Masimo SET technology platform. Now, in addition to better technology across the board, our staff no longer has to change sensors or monitors when patients are transferred between departments."
Aurora Health Care is a not-for-profit Wisconsin health care provider and a national leader in efforts to improve the quality of health care. Aurora offers care at sites in more than 90 communities throughout eastern Wisconsin, including 13 hospitals, more than 100 clinics and over 130 community pharmacies. Prior to their system-wide conversion, hospitals and surgery centers in the Aurora Health Care system struggled with pulse oximetry technology and sensor incompatibility issues.
"I've been sold on Masimo SET pulse oximetry ever since I first saw that it worked accurately on the tiniest of fingers and under the most difficult of patient conditions," stated Sharon O'Hara, BSN, RN, CAPA, Perianesthesia Supervisor, PACU, Aurora BayCare. "In this practice setting, SpO2 is the most important parameter. It can also be difficult to measure under typical PACU patient conditions, which can include hypothermia and shivering. Masimo SET eliminates artifact so we get accurate data before treating the patient."
By making the conversion to Masimo, Aurora Health Care joins many of the top hospitals in the United States—including four of the top five—as listed on the US News & World Report Honor Roll, which have all adopted Masimo SET as their primary pulse oximetry platform. Masimo SET has been clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 measurements, even under the most difficult clinical conditions such as patient motion or low peripheral perfusion. These studies demonstrate that Masimo SET delivers improvements in outcomes, safety and efficiency.
In addition to the clinical benefits realized by the adoption of Masimo SET pulse oximetry technology, Aurora officials noted that the level of service and support they received from the Masimo implementation team throughout the multi-facility conversion process impressed them. "I must admit that I was not looking forward to a system-wide technology conversion involving 13 hospitals and three surgery centers," stated Gresch. "But from the very start, the service, training and support we received from the entire Masimo clinical implementation team was spectacular."
"Masimo's implementation plan was so detailed, carefully orchestrated and executed in such a professional manner that there were absolutely no surprises," continued Gresch. "In addition, they were there whenever we needed them, answering every question we had about the process or the equipment, and providing our entire team with a solid understanding of the technology and its clinical application."
As part of the conversion, hospitals in the Aurora Health Care network will also have the ability to noninvasively measure many blood constituents with Masimo Rainbow SET Pulse CO-Oximetry that previously required invasive procedures. Masimo Rainbow SET is the first-and-only technology platform that continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO), methemoglobin (SpMet), and PVI, in addition to oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate. Developed as a scalable and upgradeable technology platform, Masimo Rainbow SET enables clinicians and hospitals to do more with their Masimo devices by building and expanding the noninvasive measurements, capabilities and features within the platform. This allows hospitals to make an investment in patient safety today that won't become obsolete tomorrow.
Rick Fishel, President of Masimo Americas, stated, "Aurora Health Care is a nationally recognized healthcare leader with a successful approach to enhancing heath care quality using a combination of demonstrated best practices and patient-centered care management. As a medical technology innovator, Masimo shares this vision, and we are proud to provide innovative solutions that can facilitate Aurora's plan to create better care processes for all of their patients. Masimo SET pulse oximetry and Masimo Rainbow SET Pulse CO-Oximetry solutions provide noninvasive capabilities that can help Aurora advance patient safety and improve the way care is delivered in a variety of clinical settings."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that Masimo SET and Masimo Rainbow SET will deliver a sufficient level of clinical improvement over alternative pulse oximetry and noninvasive patient monitoring systems to allow for further adoption of the technology at other hospitals, risks related to our assumption that this agreement with Aurora Health Care will serve to substantially increase revenues, and risks related to our assumption that Masimo's new noninvasive measurements—total hemoglobin (SpHb™) and oxygen content (SpOC™)—will deliver a sufficient level of clinical improvement over alternative hemoglobin testing capabilities to allow for rapid adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our annual report on Form 10-Q for the quarter ended March 29, 2008, filed with the Securities and Exchange Commission on May 1, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our annual report on Form 10-Q for the quarter ended March 29, 2008, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Study Demonstrates Masimo's Noninvasive PVI® Measurement Can Predict Fluid Responsiveness During Surgery
Publication in 'British Journal of Anaesthesia' reveals PVI has similar or better accuracy compared to multiple invasive methods
Irvine, California – July 10, 2008 –Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced that a new clinical study, published in the June 2008 issue of the British Journal of Anaesthesia, concluded that under the study protocol Masimo's PVI measurement "can predict fluid responsiveness in mechanically-ventilated patients under general anesthesia." PVI is a new measurement available in the Masimo Rainbow SET technology platform that allows noninvasive, automated, and continuous monitoring of the variation in the pulse oximeter waveform amplitude during respiration.
During surgery, one of the biggest challenges anesthesiologists face is deciding whether to administer fluid to increase cardiac index (amount of blood the heart pumps each minute) and maintain an adequate delivery of oxygen to the body. However, multiple studies have shown that traditional invasive measurements are only 50 to 60% accurate at predicting an improvement in cardiac index after volume administration.1 Administration of unnecessary fluid should be avoided because it is associated with increased morbidity and mortality.2 Newer invasive measurements that are based on dynamic changes during the respiratory cycle have shown improved accuracy at predicting fluid responsiveness, but market adoption has been slow due to complexity and cost. Therefore, a noninvasive method to accurately assess fluid responsiveness and guide fluid administration would provide tremendous value to clinicians and patients alike.
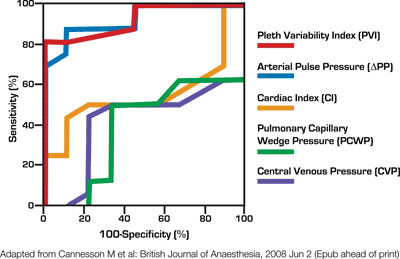
- Figure 1. Receiver Operator Characteristic (ROC) Curve for Diagnostic Accuracy of PVI Compared to Invasive Parameters.3
"In the British Journal of Anaesthesia study, a patient was defined as a responder" if their cardiac index increased by 15% or more after administration of 500 ml of fluid. If cardiac index increased less than 15% after volume administration, the patient was defined as a "non-responder". Of the 25 patients evaluated in the study, 16 were considered responders and 9 were considered non-responders. PVI showed a similar accuracy (0.93 area under the curve, AUC) at predicting fluid responsiveness when compared to pulse pressure variation from an invasive arterial catheter (0.94 AUC) and superior accuracy when compared to central venous (0.42 AUC) or pulmonary capillary wedge pressure (0.40) (Figure 1).3
"PVI demonstrated high accuracy in discriminating fluid responders from non-responders—providing a unique opportunity to better manage a patient's fluid volume to optimize cardiac performance and organ perfusion," stated Maxime Cannesson, M.D., lead researcher and anesthesiologist, Louis Pradel Hospital in Lyon, France. "14 of the 16 patients who responded to fluid administration with a cardiac index increase of 15% or more had a PVI value of >14% before volume administration. All 9 patients who did not respond to fluid administration with a cardiac index increase of 15% or more had a PVI value of <14% before volume administration."
According to a recent clinical study published in Critical Care, monitoring pulse pressure variation with an arterial catheter showed an ability "to decrease the duration of hospital stay, mechanical ventilation, and post-operative morbidity in patients undergoing high-risk surgery".4 While pulse pressure variation from an arterial pressure catheter is considered the gold standard for assessing fluid responsiveness, only a small minority of patients receive this type of catheter during surgery due to its invasiveness, complexity, and risk. In Dr. Cannesson's study, Masimo PVI showed similar accuracy as pulse pressure variation at predicting fluid responsiveness but offers clinicians an easy and noninvasive assessment of fluid responsiveness in any patient undergoing surgery by simply using their existing noninvasive Masimo SpO2 sensor with Masimo Rainbow SET monitors.
Joe E. Kiani, Chairman and CEO of Masimo, stated "The excellent work of Dr. Cannesson and colleagues adds to the growing body of evidence supporting the use of PVI as a new standard of care to optimize fluid status in patients both in the operating room and at the bedside. We are happy to see that yet another one of our inventions has the potential to improve outcomes and increase safety for patients."
1 Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002 Jun;121(6):2000-8.
2 Joshi G. "Intraoperative Fluid Restriction Improves Outcome After Major Elective Gastrointestinal Surgery." Anesthesia Analgesia (2005) 101:601-5.
3 Cannesson M, Desebbe O, Rosamel P, Delannoy B, Robin J, Bastien O, Lehot JJ. "Pleth variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre." British Journal of Anaesthesia 2008; 0:aen133v1-7.
4 Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO Jr, Michard F. "Goal-directed Fluid Management Based on Pulse Pressure Variation Monitoring During High-risk Surgery: A Pilot Randomized Controlled Trial." Critical Care 2007; 11: R100.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that the positive results and clinical outcomes presented on PVI will be repeated in other studies, risks related to our assumption that PVI will deliver a sufficient level of clinical improvement over alternative methods of monitoring respiratory variations in the pulse oximetry plethysmographic waveform amplitude to allow for rapid adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended March 29, 2008, filed with the Securities and Exchange Commission on May 1, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended March 29, 2008, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


First Nursing Facility Installation of Masimo Patient SafetyNet™ Goes Live with "Remarkable" Results
State-of-the-art, remote monitoring and clinician notification system helps keep nursing facility patients safe and clinicians happy
Irvine, California – June 26, 2008 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced the first installation of the Masimo Patient SafetyNet remote monitoring and clinician notification system outside of a hospital setting. The East Tennessee Respiratory Center at Hillcrest North, a skilled nursing facility in Knoxville, TN, installed the Patient SafetyNet system after Respiratory Support Services (RSS)—the center's respiratory care providers—outlined the significant clinical benefits that were possible with the system.
"Masimo Patient SafetyNet has made a remarkable difference in almost every aspect of respiratory care at the Hillcrest North facility," stated Gene Gantt, Chief Executive Officer of RSS. "Evidence of Masimo Patient SafetyNet's effect on patient safety is undeniable. After installation, we immediately noticed a reduction in false alarms by more than 60 percent and a marked reduction in hospital readmissions and acute care transfers, while the effectiveness of our ventilator weaning program and our clinical efficiency has improved."
Skilled nursing facilities are private institutions that present a unique challenge to patient monitoring. There are no glass windows to make observation of a patient in the privacy of their own room easy. Without an appropriate remote monitoring and clinician notification system, physiological early warning indicators—such as decreases in oxygen saturation and pulse rate, which often precede respiratory distress or cardiac arrest—may go unobserved.
"In this setting, it is difficult to adequately monitor patients without compromising their privacy or comfort," said Scott Gantt, Chief Operating Officer of RSS. "Masimo Patient SafetyNet allows us to monitor patients remotely and provides early warning alarms that tell us when the physiological parameters we have set for the patient are violated. This enables clinicians to immediately respond to the patient's bedside and initiate care before a potentially more severe event occurs."
Combining the Measure-Through-Motion-and-Low-Perfusion performance of Masimo Rainbow SET Pulse CO-Oximetry technology with wireless clinician alerts via pager, Patient SafetyNet provides a new level of safety to patients in settings that preclude the level of direct surveillance required and recommended to preempt adverse events. Masimo Rainbow SET Pulse CO-Oximetry is the first-and-only technology platform capable of continuously and noninvasively measuring many blood constituents that previously required invasive procedures, including total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO™), methemoglobin (SpMet™), pleth variability index (PVI), oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate. At the heart of the system is Masimo SET pulse oximetry, clinically proven to have the highest sensitivity (98 percent) and specificity (97 percent) through conditions of motion and low perfusion.1
"We are very excited about the Masimo Patient SafetyNet system and the improvements in care that we have already realized since implementing the system at Hillcrest North," stated Carolyn Pointer, BSN, MPH, NHA, President and CEO of Hillcrest HealthCare Communities. "In fact, we look forward to expanding the benefits of Patient SafetyNet to other facilities within our network and continuing to lead the long-term care arena in advancements in technology, monitoring and patient care improvements."
Rick Fishel, President of Masimo Americas, stated "Patient safety and quality of care are critical success factors in any inpatient setting. This installation illustrates the impact and value of Masimo Patient SafetyNet beyond the hospital setting. Respiratory Support Services and Hillcrest North are pioneering the use of this advanced patient monitoring technology in long-term care facilities with results that not only show improvements in patient safety, but also in patient outcomes. We applaud them for their vision, leadership and commitment to making a difference in the care of patients."
1 Shah N, Estanol L. " Impact of Motion and Low Perfusion on SpO2 & Pulse Rate in Three New Generation POs in Volunteers." Anesthesiology 2006; 105: A1433.
About Respiratory Support Services (RSS)
RSS is a full service post-acute care network providing respiratory therapy (RT) personnel, equipment and supplies, as well as consultancy and management services, to facilities like Hillcrest North in five southern states. In addition, RSS has successfully lead efforts to add continuous pulse oximetry monitoring to the Tennessee Standards for Ventilator Care in Rehabilitation Facilities and the American Association for Respiratory Care (AARC) Quality Respiratory Care Recognition Program.
About Hillcrest HealthCare
Hillcrest HealthCare offers services in four locations throughout Knox County, Tennessee. Hillcrest North, the largest campus with two buildings and 271 licensed beds, provides both skilled and long-term care. Hillcrest West and Hillcrest South are long-term care facilities with 194-beds and 95-beds, respectively, and LakeBrook Place is a 28-bed assisted-living facility.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring te chnologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that the positive results and clinical outcomes represented by Respiratory Support Services at the Hillcrest North Skilled Nursing Facility will be repeated at other hospitals, Masimo Patient SafetyNet will deliver a sufficient level of clinical improvement over alternative remote monitoring systems to allow for rapid adoption of the technology on general care floors, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended March 29, 2008, filed with the Securities and Exchange Commission on May 1, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended March 29, 2008, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Dartmouth-Hitchcock Medical Center Evaluation Findings Demonstrate Masimo Patient SafetyNet™ Delivers Improvements in Clinical Outcomes and Patient Safety on General Care Floors
Early results show positive impacts on safety, quality of care, clinician efficiency and nursing satisfaction
Irvine, California – May 28, 2008 – Dartmouth-Hitchcock Medical Center and Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced that initial results of an ongoing clinical evaluation show Masimo Patient SafetyNet with Masimo Rainbow SET Pulse CO-Oximetry provides early warning detection of impending patient deterioration on the general care floor, which helped keep patients safer. Early findings showed a reduction in distress codes, rescue activations, and ICU transfers. Dartmouth-Hitchcock Medical Center (DHMC), the first hospital to deploy the Masimo Patient SafetyNet system, presented its evaluation data and findings to more than 1,500 clinicians at the 4th International Conference on Rapid Response Systems on May 8, 2008 in Toronto, Canada, and the National Patient Safety Foundation (NPSF) Annual Congress on May 15, 2008 in Nashville, TN.
 |
George T. Blike, M.D., Medical Director, Patient Safety, DHMC, stated "The data that we have gathered by continuously monitoring all patients on a post-surgical general care floor since beginning this evaluation in late 2007 confirms what we anticipated—an improvement in prevention and intervention. The Masimo Patient SafetyNet system makes it possible for our clinicians to identify patient distress earlier—preventing codes and rescues—and initiate appropriate intervention more rapidly to improve patient outcomes, recoveries and clinician efficiencies. All of this contributes to a safer general care floor for our patients."
Findings presented by DHMC showed an 80 percent decrease in distress codes and rescue activations and a 50 percent decrease in ICU transfers for Masimo Patient SafetyNet system-monitored patients in a 36-bed post-surgical unit. In addition, data gathered during the three-month evaluation, covering 2,587 total patient days, showed that the Masimo Patient SafetyNet system supported the early identification of patients with sedation or analgesia-induced respiratory depression, cardiac anomalies identified by high and low pulse rate, poor heart rate control, acute bradycardia needing atropine, new onset A-fib, unrecognized obstructive patterns of respiration like sleep apnea, and pulmonary complications such as fat emboli syndrome, pulmonary embolus and edema.
"The bottom line is that the earlier patient distress is discovered and intervention is initiated, the better the recovery will be—enabling patients to get better faster and go home quicker," said Dr. Blike. "The decreases in codes, rescue activations and ICU transfers are crucial indicators that we are catching deteriorations much earlier and that patients are safer because of it. We expect that our continued evaluation efforts will yield more financial and statistical data concerning Patient SafetyNet's impact on length of stays, patient throughput and new diagnosis identifications."
An influx of more acute patients, the growth and use of patient-controlled analgesia, an increasing number of patients with obstructive sleep apnea, and the ongoing clinician shortage are the realities of today's general care floors. As a result, patients on general care floors are at increased risk of un-witnessed adverse physiological events, like respiratory distress or cardiac arrest. If the patient is not monitored reliably using pulse oximetry technology with high specificity and sensitivity, adverse events on the general care floors may result in long-term health complications or death. Only Masimo Rainbow SET Pulse CO-Oximetry technology provides the greatest sensitivity, at 98 percent, and the greatest specificity, at 97 percent, based on independent and objective references that have examined oximeter performance in real clinical environments.
The Masimo Patient SafetyNet system combines the Measure-Through-Motion-and-Low-Perfusion performance of Masimo Rainbow SET Pulse CO-Oximetry technology with wireless clinician alerts via pager to provide a new level of safety to patients on general care floors, where nurse-to-patient ratios preclude the level of direct surveillance required and recommended to preempt adverse events. When physiological parameters are violated, such as arterial blood oxygen saturation or pulse rate, Masimo Patient SafetyNet provides accurate, actionable alarm notification directly to a qualified clinician—enabling immediate notification and early intervention.
While previous attempts at monitoring patients on the general care floors have failed because of excessive false alarms, the advanced technology of Masimo SET pulse oximetry has virtually eliminated false alarms while delivering the most accurate and reliable arterial blood oxygen saturation and pulse rate measurements. Clinically-proven in more than 100 independent and objective studies to have the highest sensitivity and specificity through conditions of motion and low perfusion—the most common source of false alarms with other pulse oximetry technologies—Masimo SET provides the foundational technology for Masimo Rainbow SET Pulse CO-Oximetry.
"Our data shows overall alarm rates of 4.1 per patient per day on our post-surgical general care floor," said Jean Avery, MBA,RN, Clinical Improvement, DHMC. "On an unmonitored patient, many of these alarm conditions would have gone unnoticed."
"One major East Coast hospital that tried to monitor patients continuously on the general care floor before the advent of Masimo SET pulse oximetry experienced about 36,000 alarms per month for 12 beds, which translated to about 96 alarms per patient day," said Jim Welch, Vice President of Patient Safety Initiatives at Masimo. "The high rate of false alarms due to conventional pulse oximetry's limitations made monitoring on the general floor impractical, if not impossible. With just 4 alarms per patient per day at DHMC, Masimo's revolutionary Measure-Through-Motion pulse oximetry finally enables accurate and reliable monitoring on general care floors."
Also, as part of the evaluation, DHMC surveyed nursing staff in the unit equipped with Masimo Patient SafetyNet and found that they were overwhelmingly satisfied with the system. When asked to assess their feelings toward the new surveillance system, the unit's nursing staff "strongly agreed" that the ability to provide surveillance has added benefit in providing safe care to patients. In addition, the nursing staff indicated that they would "definitely" want a friend or relative needing inpatient care to come to the unit equipped with a surveillance monitoring system..
"Our nurses are discovering patients in need of attention, much sooner than before," stated Nancy Karon, BSN, RN, Clinical Coordinator, DHMC. "Patient SafetyNet has improved patient care with high levels of nurse satisfaction because they know they are able to deliver the best possible quality of care to their general care floor patients."
Joe E. Kiani, Chairman and CEO of Masimo, stated "Quite simply, we developed Masimo Patient SafetyNet to help clinicians save lives and improve patient outcomes. The empirical and statistical data being gathered through Dartmouth-Hitchcock Medical Center's evaluations are providing the necessary details that demonstrate how Masimo's Measure-Through-Motion pulse oximetry technology along with the Patient SafetyNet system are impacting critical patient safety benchmarks, such as the number of sentinel events, codes, rapid response team activations and ICU transfers. Ranked as one of the top academic medical centers in the U.S. and named as one of the nation's most wired medical centers by Hospitals and Health Networks magazine, DHMC was the ideal pilot installation for Masimo Patient SafetyNet. These findings support the necessity and value of Masimo Patient SafetyNet on general care floors."
A study published in the Journal of Critical Care Medicine suggests that there are approximately 820,000 unmonitored beds in non-critical care settings in the U.S.
About Dartmouth-Hitchcock Medical Center
Nationally-ranked as one of the top academic medical centers, Dartmouth-Hitchcock Medical Center provides comprehensive inpatient and outpatient services to patients from throughout New England. With more than 21,500 inpatient admissions, 28,700 emergency department visits, over 1,699,500 outpatient visits and the region's only Level I Trauma Center, Dartmouth-Hitchcock Medical Center includes: Mary Hitchcock Memorial Hospital, Dartmouth-Hitchcock Clinic, Dartmouth Medical School, the Veterans Affairs Medical Center in White River Junction, VT., Norris Cotton Cancer Center (a National Cancer Institute-designated Comprehensive Cancer Center), Children's Hospital at Dartmouth (CHaD) and more than 50 outpatient clinics and practices.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that the positive results and clinical outcomes presented by Dartmouth-Hitchcock Medical Center will be repeated at other hospitals, Masimo Patient SafetyNet will deliver a sufficient level of clinical improvement over alternative remote monitoring systems to allow for rapid adoption of the technology on general care floors, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended March 29, 2008, filed with the Securities and Exchange Commission on May 1, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended March 29, 2008, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.

Masimo Receives FDA Clearance for Noninvasive Total Hemoglobin
Irvine, California – May 14, 2008 – Masimo, the inventor of Pulse CO-Oximetry™ and Measure-Through Motion-and-Low-Perfusion pulse oximetry, today announced it has received FDA clearance for its breakthrough noninvasive and continuous total hemoglobin monitoring technology (SpHb™). The availability of Masimo SpHb technology should make hemoglobin measurement more convenient and broadly available to clinicians in both hospital and outpatient settings—helping them make earlier and better clinical decisions, improve patient safety and decrease costs. Noninvasive total hemoglobin will be offered as part of the upgradable Masimo Rainbow SET technology platform.
Ronald Miller, MD, Professor and Chair of the Department of Anesthesia and Perioperative Care at the University of California at San Francisco (UCSF) stated, "Management of appropriate blood levels is vitally important to sustain life. Without up-to-date hemoglobin levels, patient bleeding in the operating room, recovery room, intensive care or trauma departments—where blood loss is common—can often go undetected until it poses critical short and long-term dangers to health and recovery. The ability to immediately and continuously measure hemoglobin levels will facilitate the timely administration of appropriate blood products. Conversely, during surgery, because blood is a precious and costly resource, continuously measuring hemoglobin levels noninvasively can help clinicians avoid unnecessary blood transfusions and decrease costs by more effectively titrating blood and blood replacement products."
Joe E. Kiani, Chairman and CEO of Masimo, said, "Developing breakthrough technologies that enhance patient safety and automate patient care is a responsibility that we take seriously. With noninvasive hemoglobin as part of the upgradable Masimo Rainbow SET platform, we are proud to be revolutionizing the way clinicians can assess anemic status and make more timely decisions that affect millions of patients worldwide."
The need for better hemoglobin monitoring to manage blood levels is reinforced by recently published controlled studies that show the safety of blood transfusions can be improved by the use of transfusion thresholds. In a 2008 study by the Cochrane Collaboration titled Transfusion Thresholds and Other Strategies for Guiding Allogeneic Red Blood Cell Transfusion, reviewers examined evidence from ten trials—reporting outcomes on a total of 1,780 patients—and found that "restrictive transfusion strategies reduced red blood cell transfusions by 42%."
Additionally, while noting that not all of these results were statistically significant and that additional studies are required to confirm the findings, the Cochrane reviewers also reported that, "on average, mortality was 20% lower with the restrictive compared with the liberal transfusion triggers." Similarly, five of the ten studies examined showed a reduction in hospital length of stay, while three showed a reduction in ICU length of stay.1
Continuous, noninvasive hemoglobin monitoring with Masimo Rainbow SET SpHb may enable more restrictive transfusion triggers and help maintain optimal hemoglobin levels for critically-ill patients. In addition to facilitating better blood level management, Masimo Rainbow SET's noninvasive hemoglobin monitoring capability should also help clinicians better manage chronic anemia, a blood disorder affecting two billion people worldwide that is one of today's most prevalent public health problems. Masimo Rainbow SET SpHb should provide hospitals, emergency medical professionals, dialysis centers, family physicians, cardiologists, pediatricians and other care providers with a more convenient and accessible way to manage this pervasive condition.
More than 350 million invasive hemoglobin lab tests are performed each year in the U.S. alone, making it one of the most common laboratory tests. Hemoglobin lab tests are costly, time-consuming and require that clinicians use a needle to draw a patient's blood—however, they only provide delayed and intermittent data. Masimo's SpHb technology requires no invasive procedures and provides continuous, real-time, pain-free results. This should allow clinicians to perform fewer lab tests, better manage blood transfusions and hemodialysis procedures, speed detection of internal bleeding, and more efficiently assess chronic anemia—all of which should help improve patient outcomes and reduce the cost-of-care.
"No other technology can provide continuous, noninvasive hemoglobin measurements," stated Michael O'Reilly, MD, Executive Vice President of Medical Affairs at Masimo. "Masimo Rainbow SET SpHb has the potential to revolutionize the management of both acute and chronic anemia, as well as the therapeutic interventions used to treat these conditions. We believe SpHb will provide a significant advancement in patient safety for clinicians worldwide."
The Masimo Rainbow SET platform allows clinicians to noninvasively and continuously measure oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI® and total hemoglobin (SpHb™), in addition to oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). Masimo anticipates commercial availability for both SpHb and SpOC in Q3 2008 to select customers.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that Masimo's new noninvasive measurements—total hemoglobin (SpHb™) and oxygen content (SpOC™)—will deliver a sufficient level of clinical improvement over alternative hemoglobin measurement capabilities to allow for rapid adoption of the technology and risks related to our assumptions regarding the timing or commercial availability of SpHb and SpOC, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended March 29, 2008, filed with the Securities and Exchange Commission on May 1, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended March 29, 2008, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
1 Hill SR, Carless PA, Henry DA, Carson JL, Herbert PC, McClelland DBL, Henderson KM. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2000, Issue 1. Art. No.: CD002042. DOI: 10.1002./14651858.CD002042.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Saint Barnabas Medical Center Converts to Masimo SET Technology – Establishing a Foundation for the Future of Pulse Oximetry
Hospital-wide conversion achieves technology and sensor standardization, creates process efficiencies and reduces costs
Irvine, California—May 9, 2008 -- Saint Barnabas Medical Center and Masimo, the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion Pulse Oximetry, have announced the completion of Saint Barnabas Medical Center's hospital-wide implementation of Masimo SET pulse oximetry. The New Jersey-based hospital performed an extensive comparison and thorough evaluation spanning virtually every department and cited superior performance, technology capabilities and future clinical application as chief reasons for converting to Masimo SET.
"The decision to convert to Masimo SET pulse oximetry was multifaceted, with numerous benefits that we didn't think were possible in one technology," said Shyan Sun, M.D., Director of Neonatology, Saint Barnabas Medical Center. "With Masimo, we now have a standardized technology for oximetry that not only enables sensor consistency and standardization across departments at reduced costs, but also provides the system compatibility necessary to track oximetry inventory and costs – all in a technology platform that is uniquely expandable to support our current and future oximetry needs."
Saint Barnabas Medical Center is the flagship of the Saint Barnabas Health Care System, New Jersey's largest integrated health care delivery system, and ranks among the top 5 percent of all hospitals in the country. The 601-bed institution treats more patients annually and delivers more babies than any other facility in New Jersey, including more than 40,000 inpatients admissions, over 65,000 Emergency Department visits, and nearly 7,000 births each year. Additionally, the Medical Center and its Ambulatory Care Center provide treatment and services for more than 300,000 outpatient visits annually. The Saint Barnabas Health Care System includes six acute care hospitals, a behavioral health center, ambulatory care facilities, eight nursing and rehabilitation centers, an assisted living facility, geriatric centers, a state-wide behavioral health network, and comprehensive home care and hospice programs.
Prior to their conversion, Saint Barnabas had a handful of various oximetry monitors and technologies that each required different sensors from different vendors—eliminating the ability to purchase sensors in combined volume. On top of this, these varying technologies were incompatible with the hospital's inventory management program software and could not provide usable data to track inventory movement and oximetry costs. Converting to Masimo SET pulse oximetry helped Saint Barnabas Medical Center to overcome these challenges, while reducing costs and creating process efficiencies that help to improve the process of care.
In addition to Saint Barnabas's hospital-wide standardization to Masimo SET pulse oximetry, the conversion also allows Saint Barnabas clinicians to utilize Masimo Rainbow SET technology – an upgradable noninvasive blood constituent monitoring platform that is capable of measuring additional blood constituents that previously could only be measured with invasive procedures. Masimo Rainbow SET is the first and only method to continuously and noninvasively measure total hemoglobin (SpHb™) and oxygen content (SpOC™) (both pending regulatory clearances), carboxyhemoglobin (SpCO) and methemoglobin (SpMet), in addition to oxyhemoglobin (SpO2), perfusion index (PI), pleth variability index (PVI) and pulse rate. Developed as a scalable and upgradeable technology platform, Masimo Rainbow SET enables clinicians and hospitals to do more with their Masimo devices by building and expanding the noninvasive measurements, capabilities and features within the platform. This allows hospitals to make an investment in patient safety today that won't become obsolete tomorrow.
By making the conversion to Masimo, Saint Barnabas joins many of the top hospitals in the United States—including four of the top five—as listed on the US News & World Report Honor Roll, which have all adopted Masimo SET as their primary pulse oximetry platform. Clinically proven in more than 100 independent and objective studies, Masimo SET provides the most trustworthy SpO2 readings, even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. These studies demonstrate that Masimo SET delivers improvements in outcomes, safety and efficiency.
Joe E. Kiani, CEO of Masimo, stated, "Saint Barnabas Medical Center's conversion to Masimo SET pulse oximetry ensures they have the best of the present and are set for the future with the upgradeable Masimo Rainbow SET technology platform. They represent a growing industry trend toward pulse oximetry technologies that have value and relevance into the future. Masimo Rainbow SET is the first pulse oximetry technology platform to pioneer this capability and the only one that can provide the innovation to drive this proposition for hospitals worldwide. We value Saint Barnabas for their commitment to patient care, quality and this vision."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™) and oxygen content (SpOC™) (both pending regulatory clearances), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that Masimo SET and Masimo Rainbow SET will deliver a sufficient level of clinical improvement over alternative pulse oximetry and noninvasive patient monitoring systems to allow for further adoption of the technology at other hospitals, risks related to our assumption that this agreement with Saint Barnabas will serve to substantially increase revenues, and risks related to our assumption that Masimo's new noninvasive measurements—total hemoglobin (SpHb™) and oxygen content (SpOC™)—will deliver a sufficient level of clinical improvement over alternative hemoglobin testing capabilities to allow for rapid adoption of the technology and risks related to our assumptions regarding the timing or commercial availability of SpHb and SpOC, and will be timely cleared, if ever, by appropriate regulatory bodies, as well as other factors discussed in the "Risk Factors" section of our annual report on Form 10-Q for the quarter ended March 29, 2008, filed with the Securities and Exchange Commission on May 1, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our annual report on Form 10-Q for the quarter ended March 29, 2008, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Showcases Best-in-Class Patient Safety Solutions at the AACN Conference
Masimo introduces the new Rad-87 Pulse CO-Oximeter, Patient SafetyNet, and noninvasive hemoglobin
Irvine, California—May 6, 2008 -- Masimo, the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion Pulse Oximetry, will showcase the latest technology for patient safety solutions at the American Association of Critical Care Nurses (AACN), National Teaching Institute (NTI) & Critical Care Exposition in Chicago on May 6-8, 2008. Live demonstrations of the new Masimo Rad-87 Pulse CO-Oximeter™, Masimo Patient SafetyNet™ and continuous, noninvasive hemoglobin (SpHb™) (pending FDA clearance) will show how Masimo technologies can help critical care clinicians advance patient safety, improve patient outcomes, and increase clinical efficiencies.
This year, the Joint Commission, an independent, not-for-profit organization that accredits and certifies more than 15,000 health care organizations and programs in the United States, recognized improved recognition and response to changes in a patient's condition as one of their key National Patient Safety Goals.
A key foundational component to improving patient safety on the general floor is the unprecedented sensitivity and specificity of Masimo SET pulse oximetry. Before Masimo SET, pulse oximeters were reported to falsely alarm more than 90% of the time. In fact, one major hospital attempted to monitor patients on the general floor before the advent of Masimo SET and reported thousands of false alarms per month.
Another key component to improving clinician response to changing patient conditions on the general care floor is Masimo Patient SafetyNet—a new easy-to-use remote monitoring and clinician notification system that reliably and cost-effectively delivers patient alarms to assigned clinicians. Combining the performance of Masimo SET® pulse oximetry with wireless clinician notification via pager, Patient SafetyNet provides a new level of safety to patients on general care floors.
A perfect complement to Masimo SET and Patient SafetyNet is the new Masimo Rad-87 bedside monitor. The Rad-87 is an easy-to-use, yet fully-featured Pulse CO-Oximeter with a built-in 802-11a/b/g radio for bidirectional wireless communication with the Patient SafetyNet system. Recently cleared by the FDA, it features a simple, intuitive user-interface design with an easy-to-read, high-contrast display that allows clinicians to clearly see the Masimo Rainbow SET measurements—even from across the room. Alarms and alerts can be enabled at the bedside or remotely, via the Patient SafetyNet system. Rad-87 allows activation of many features with only a single touch and its unique visual display allows users to quickly confirm if the alarm settings are appropriate for the patient environment.
Rad-87 also features Masimo Rainbow SET, the first-and-only noninvasive blood constituent monitoring platform measuring carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate. A cost-effective solution for critical care customers who value ease-of-use and versatility, Rad-87 will also be capable of displaying Masimo's newest Rainbow measurement—noninvasive total hemoglobin (SpHb™) (pending FDA clearance).
As the Anesthesiology Patient Safety Foundation (APSF) has recommended, continuous monitoring of oxygenation (pulse oximetry) and ventilation should reduce the incidence of preventable postoperative death and injury on the general care floors.1 Rad-87 and Patient SafetyNet should help hospitals provide the level of care their patients on the general floors need without the burden of excessive false alarms on their already stretched resources.
In addition, Rad-87's ability to provide noninvasive and continuous hemoglobin monitoring should help improve patient outcomes and reduce the cost of care by providing real-time anemia monitoring—potentially allowing clinicians to perform fewer lab tests, better manage blood transfusions, speed detection of internal bleeding, and more efficiently assess chronic anemia.
Joe E. Kiani, CEO of Masimo, stated, "Masimo has had a longstanding commitment to 'do what is best for patient care' through innovation. The new Rad-87, Patient SafetyNet and Rainbow SET technologies that we are presenting to the critical care community at the AACN underscore our commitment and highlight our continued focus on developing innovative monitoring solutions to empower healthcare professionals to provide more advanced and comprehensive care to their patients."
Sponsored by the American Association of Critical Care Nurses, the NTI & Critical Care Exposition is the largest and most comprehensive trade show for acute and critical care nurses.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™) and oxygen content (SpOC™) (both pending regulatory clearances), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that Masimo Patient SafetyNet, Rad-87, Masimo SET and Masimo Rainbow SET technologies will deliver a sufficient level of clinical improvement to allow for further adoption of pulse oximetry or Pulse CO-Oximetry on the general care floors and risks related to our assumption that Masimo's new noninvasive measurements—total hemoglobin (SpHb™) and oxygen content (SpOC™)—will deliver a sufficient level of clinical improvement over alternative invasive testing capabilities to allow for rapid adoption of the technology and risks related to our assumptions regarding the timing or commercial availability of SpHb and SpOC, and will be timely cleared, if ever, by appropriate regulatory bodies, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended March 29, 2008, filed with the Securities and Exchange Commission on May 1, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended March 29, 2008, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
1 Anesthesia Patient Safety Foundation (ASPF) Initiatives: "Safety During Patient-Controlled Analgesia"; October 13, 2006. http://www.apsf.org/initiatives_safety.php
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, PVI, Radical-7, Rad-87, Rad-57,Rad-9, Rad-8, Rad-5,Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
Masimo will preview the new Masimo Rad-87 at this year's NTI. The Rad-87 is an easy-to-use, yet fully-featured Pulse CO-Oximeter with a built-in 802-11a/b/g radio for bidirectional wireless communication with the Patient SafetyNet system.


Masimo to Present at Deutsche Bank 33rd Annual Health Care Conference
Irvine, California, May 1, 2008 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry-and-Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced that it is scheduled to present at the Deutsche Bank 33rd Annual Health Care Conference at the InterContinental Boston in Boston, MA on Tuesday, May 6, 2008 at 9:30 a.m. ET. Joe E. Kiani, Chairman will be presenting.
A live webcast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the webcast will be available following the live presentation.
About Masimo
Masimo (Nasdaq: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™) and oxygen content (SpOC™) (both pending FDA clearance), carboxyhemoglobin (SpCO®), methemoglobin (SpMet® ), and pleth variability index (PVI®), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at http://www.masimo.com.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
949-297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports First Quarter 2008 Financial Results
Record results mark 19th consecutive quarter of revenue growth
Q1 2008 Highlights:
- Product revenues increased 30% to a record $59.7 million
- Shipped 28,600 new pulse oximeters
- Rainbow revenues increased 104%
Irvine, California, April 29, 2008 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced its financial results for the 2008 first quarter ended March 29, 2008.
For the first quarter of 2008, Masimo reported product revenues of $59.7 million representing a 30% increase over $45.8 million for the first quarter of 2007. Including royalty revenues, Masimo reported total 2008 first quarter revenues of $71.1 million compared to $59.0 million for the first quarter of 2007. Net income for the 2008 first quarter was $8.8 million representing $0.15 per common share compared to $9.1 million or $0.11 per common share for the first quarter of 2007.
Masimo also reported that it shipped 28,600 Masimo SET and Masimo Rainbow SET oximetry units, excluding handheld units, during the first quarter of 2008. This represented an 8% increase from 26,500 units in the comparable prior year period, resulting in a new estimated net worldwide installed base of 491,000 Masimo SET pulse oximeters.
Joe E. Kiani, Chairman and Chief Executive Officer of Masimo, said, "We are happy to report first quarter results that once again exceeded expectations. We believe that these results reflect the increasing momentum for the clinical adoption of our life saving Masimo SET and Masimo Rainbow SET technologies. In fact, in the first quarter of 2008, demand for our new Masimo Rainbow SET technologies, on the strength of our Rad-57 handheld carbon monoxide measuring device, increased over 100% compared to Q1 last year."
Cash and cash equivalents totaled $86.3 million at March 29, 2008. Masimo also reported that during the 2008 first quarter, it satisfied in full a $26.7 million debt obligation, the majority of which was originally established in early fiscal 2007.
Conference Call
Masimo will hold a conference call today at 2:00 p.m. PT (5:00 p.m. ET) to discuss the results. The dial-in numbers are (866) 831-6247 for domestic callers and (617) 213-8856 for international callers. The reservation number for both dial-in numbers is 82345350. A live webcast of the conference call will be available online from the "Investor Relations" page of the Company's corporate website at www.masimo.com.
After the live webcast, the call will remain available on Masimo's website through May 29, 2008. In addition, a telephonic replay of the call will be available until May 13, 2008. The replay dial-in numbers are (888) 286-8010 for domestic callers and (617) 801-6888 for international callers. Please use reservation code 71776300.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™) and oxygen content (SpOC™) (both pending regulatory clearances), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements. All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations, prospects and business generally; the market acceptance of our technologies and products; the value of measuring new parameters; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and expectations for total revenues, product revenues, GAAP earnings per share, non-GAAP pro forma earnings per share and stock based compensation expenses for the full fiscal year 2008. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our reliance on Masimo SET and related products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; the failure to continue developing innovative products and technologies; the lack of acceptance of any new products and technologies of ours; obtaining regulatory approval of our current and future products and technologies, including the recently announced total hemoglobin measurement; the loss of our customers the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our Annual Report on Form 10-K for the fiscal year ended December 29, 2007 filed with the Securities and Exchange Commission on March 4, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our Annual Report on Form 10-K for the fiscal year ended December 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
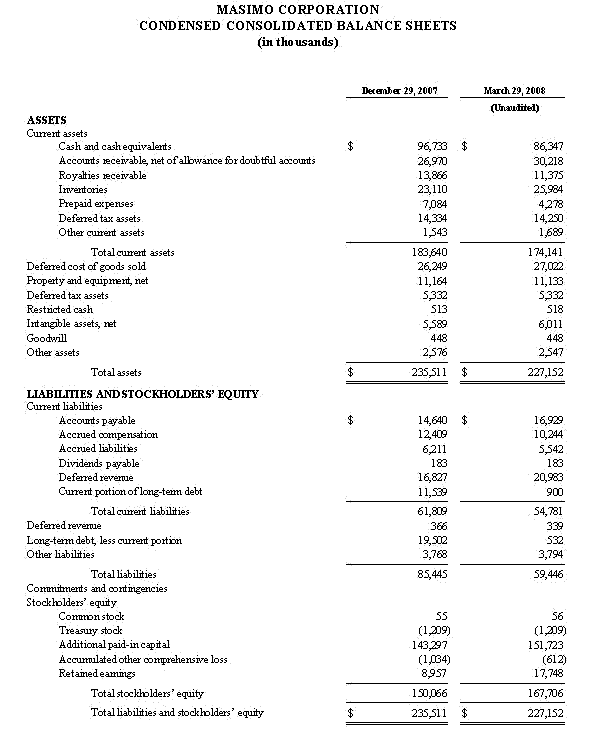
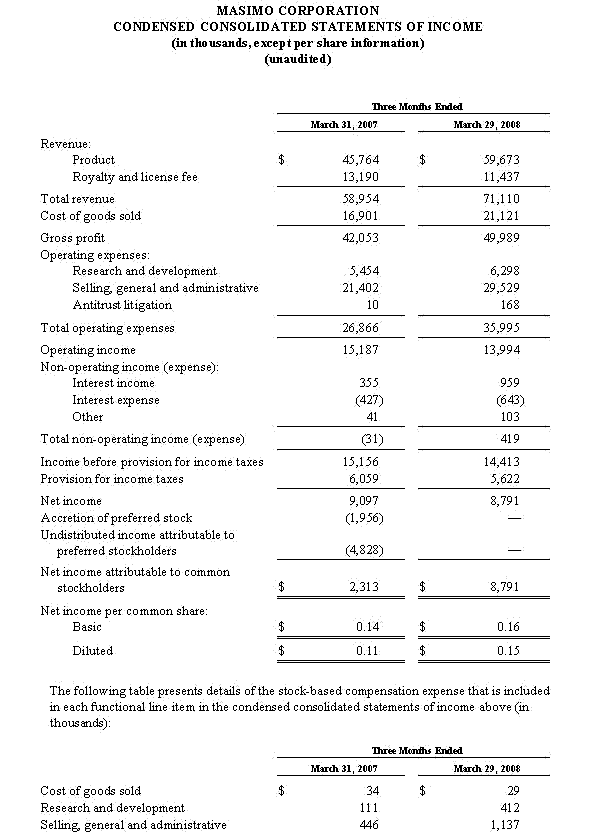

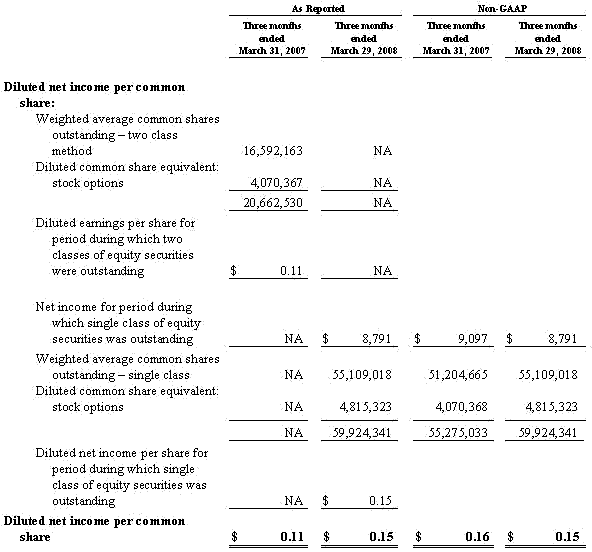


Masimo to Report First Quarter 2008 Financial Results on April 29, 2008
Conference call and webcast with Masimo CEO and CFO to begin after markets close at 2:00 p.m. PT (5:00 p.m. ET)
IRVINE, Calif., April 18, 2008—Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced it will release first quarter financial results for the period ended March 29, 2008 after the market closes on Tuesday, April 29, 2008.
A conference call to review the results will begin at 2:00 p.m. PT (5:00 p.m. ET) and will be hosted by Joe E. Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President & Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the Company's corporate website at www.masimo.com. The dial-in numbers are (866) 831-6247 for domestic callers and (617) 213-8856 for international callers. The reservation number for both dial-in numbers is 82345350. After the live webcast, the call will remain available on Masimo's website through May 29, 2008. In addition, a telephonic replay of the call will be available until May 13, 2008. The replay dial-in numbers are (888) 286-8010 for domestic callers and (617) 801-6888 for international callers. Please use reservation code 71776300.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™) and oxygen content (SpOC™) (both pending regulatory clearances), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Mark P. de Raad
Executive Vice President and
Chief Financial Officer
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Masimo Corporation
949-297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Atom Medical Adopts Masimo Rainbow SET Technology
Irvine, California – April 16, 2008 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through-Motion-and-Low-Perfusion pulse oximetry, today announced an agreement with Atom Medical Corporation, a leading global manufacturer of neonatal products and technologies, to integrate Masimo Rainbow SET Pulse CO-Oximetry into its current and future product solutions.
"Since standardizing on Masimo technology more than 10 years ago, our neonatal monitoring solutions and customers have greatly benefited from the superior performance of Masimo SET pulse oximetry," said Kazuo Matsubara, President of Atom Medical. "Now, with the groundbreaking capabilities of Masimo Rainbow SET and its ability to provide vital measurements that previously required invasive lab procedures, we are confident that the clinical community which we serve will experience even greater clinical and financial benefits from our solutions."
Masimo Rainbow SET is the first and only technology platform capable of noninvasively and continuously measuring total hemoglobin (SpHb™) and oxygen content (SpOC™)—both measurements pending FDA and other regulatory clearances—as well as carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and pleth variability index (PVI®), in addition to oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR).
Masimo Rainbow SET has the potential to help clinicians save lives through more rapid diagnosis of potentially life-threatening conditions, allowing faster treatment decisions and improved patient outcomes. The latest Masimo Rainbow SET measurement, SpHb, may enable medical personnel in both hospital and outpatient settings to quickly identify anemia or blood loss without having to draw blood or wait for lab results.
In neonatal settings, the ability to continuously and noninvasively measure a patient's methemoglobin levels is especially important due to the risks associated with the use of inhaled nitric oxide (iNO) therapy to treat hypoxic respiratory failure in newborns, which has been shown to induce methemoglobinemia. According to the American Academy of Pediatrics, "infants who receive iNO therapy should be monitored according to institutionally derived protocols designed to avoid the potential toxic effects associated with iNO administration. These effects include methemoglobinemia (secondary to excess nitric oxide concentrations), direct pulmonary injury (attributable to excess levels of nitrogen dioxide), and ambient air contamination." 1
Masimo Chairman and CEO, Joe E. Kiani, said, "Atom is a long-time Masimo partner dedicated to developing innovative solutions for the challenging neonatal clinical setting. The addition of Masimo Rainbow SET affirms their position as a leading global provider of advanced neonatal product solutions, leveraging breakthrough technologies to improve the quality of care for the tiniest of patients. We commend Atom for their commitment to patient care and product innovation."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion-and-Low-Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™) and oxygen content (SpOC™) (both pending regulatory clearances), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), pulse rate (PR), and perfusion index (PI), allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that this agreement with Atom and their adoption of Masimo technology will serve to substantially increase revenues, risks related to our assumption that Masimo's new noninvasive measurements—total hemoglobin (SpHb™) and oxygen content (SpOC™)—will deliver a sufficient level of clinical improvement over alternative hemoglobin testing capabilities to allow for rapid adoption of the technology and risks related to our assumptions regarding the timing or commercial availability of SpHb and SpOC, and will be timely cleared, if ever, by appropriate regulatory bodies, as well as other factors discussed in the "Risk Factors" section of our annual report on Form 10-K for the year ended December 29, 2007, filed with the Securities and Exchange Commission on March 4, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our annual report on Form 10-K for the year ended December 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
1 AMERICAN ACADEMY OF PEDIATRICS: Pediatrics, Vol. 106 No. 2 August 2000, pp. 344-345, Use of Inhaled Nitric Oxide, Committee on Fetus and Newborn.
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo PVI® Cleared for Market in Japan
PVI could help clinicians assess a patient's fluid responsiveness noninvasively, according to a new study published in "Anesthesia & Analgesia"
Irvine, California - April 3, 2008 - Masimo, the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, today announced Japanese Ministry of Health, Labor and Welfare (MHLW) approval of its PVI measurement. PVI is an index automatically derived from the Masimo plethysmographic waveform, which has been demonstrated to noninvasively assess fluid responsiveness in mechanically ventilated patients and can help clinicians assess if a patient's cardiac function is compromised.
PVI may help clinicians and emergency professionals to determine if a patient is dehydrated or over-hydrated—enabling more accurate fluid administration decisions—all by simply referring to the numerical Masimo PVI value that is continuously displayed on Masimo Rainbow SET Pulse CO-Oximeters. With one noninvasive sensor, Masimo Rainbow SET technology delivers multiple physiologic measurements that previously required invasive blood tests, including total hemoglobin (SpHb™) and oxygen content (SpOC™) (both pending FDA and other regulatory clearances), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, oxyhemoglobin (SpO2), perfusion index and pulse rate.
During surgery and post-operatively, the immediate identification and rapid intervention of patients who are most likely to respond to fluid administration (fluid responders) can enable organ preservation, while recognizing patients unlikely to respond to fluid administration (fluid non-responders) can prevent pulmonary edema. Clinical studies have shown that current static methods for assessing fluid responsiveness, including clinical examination, arterial blood pressure, heart rate and central venous and pulmonary artery occlusion pressure, are poor predictors of fluid responsiveness. Also dynamic indices, such as respiratory variations in arterial pulse pressure, inferior vena cava diameter, superior vena cava diameter and stroke volume, present significant limitations, are invasive and often operator-dependent.
PVI is a dynamic new indicator of fluid responsiveness that does not require an invasive procedure or manual calculation, yet has been demonstrated to be sensitive to changes in preload and to be an accurate predictor of fluid responsiveness in mechanically ventilated patients.
In this month's Anesthesia and Analgesia Journal, a clinical study titled, "Does the Pleth Variability Index (PVI) Indicate the Respiratory-Induced Variation in the Plethysmogram and Arterial Pressure Waveforms?" headed by Dr. Maxime Cannesson from the Louis Pradel Hospital and the Claude Bernard Lyon University in Lyon, France, studied 25 patients under general anesthesia and mechanical ventilation and found a strong correlation between Masimo's PVI measurement and the manually measured Delta POP (r=0.92), with a sensitivity of 93% and a specificity of 97%. The researchers had previously shown a high correlation between Delta POP and fluid responsiveness. The recent study findings also confirmed that the relationship between PVI and Delta POP was still significant when performed in the Anti-Trendelenburg (r=0.94) and Trendelenberg (r=0.93) body positions, illustrating the responsiveness of PVI to the dynamic and changing fluid volume status. Dr. Cannesson et al. concluded that PVI has "potential clinical applications for noninvasive fluid responsiveness monitoring." 1
"PVI should provide clinicians with the most effective and efficient noninvasive method of continuously measuring their patient's fluid volume," stated Michael O'Reilly, MD, EVP of Medical Affairs at Masimo. "The addition of PVI in Masimo pulse oximeters now available in Japan should help clinicians in Japan add a level of certainty and immediacy toward balancing and managing intravascular fluid volumes and cardiac output both inside and outside of the operating room."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™) and oxygen content (SpOC™) (both pending FDA and other regulatory clearances), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to oxyhemoglobin (SpO2), perfusion index and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that PVI will prove to be an effective clinical indicator of patient hydration and the need for fluid loading, that the findings in the study referred to herein will be replicated in future studies, that approval of PVI by the Japanese MHLW will serve to materially increase Masimo product sales or revenues targets for 2008, and assumptions related to the development of products and technologies that may compete with PVI, as well as other factors discussed in the "Risk Factors" section of our annual report on Form 10-K for the year ended December 29, 2007, filed with the Securities and Exchange Commission on March 4, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our annual report on Form 10-K for the year ended December 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
1 Does the Pleth Variability Index Indicate the Respiratory Induced Variation in the Plethysmogram and Arterial Pressure Waveforms? Maxime Cannesson, MD, Bertrand Delannoy, MD, Antoine Morand, MD, Pascal Rosamel, MD, Yassin Attof, MD, Olivier Bastien, MD, PhD, Jean-Jacques Lehot, MD, PhD. Department of Anesthesiology and Intensive Care, Louis Pradel Hospital and Claude Bernard Lyon University, Lyon, France. Anesthesia & Analgesia. Vol. 106. No. 4. April 2008.
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
Contact:
Dana Banks
Masimo Corporation
949-297-7348


Schiller AG Adopts Masimo Rainbow SET as their Technology Platform of Choice for Patient Monitoring Devices Worldwide
Irvine, California – March 25, 2008 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, today announced an agreement with Switzerland-based Schiller, a leading European manufacturer and supplier of electrocardiographs, spirometers, patient monitors and external defibrillators, to integrate the Masimo Rainbow SET technology platform as the foundational technology of choice for all their patient monitoring solutions worldwide.
Masimo Rainbow SET, an upgradeable noninvasive technology platform featuring the accuracy and reliability of Masimo SET Measure-Through Motion and Low Perfusion pulse oximetry, is revolutionizing patient monitoring by significantly expanding pulse oximetry's ability to capture, track and monitor blood constituents that previously required invasive procedures. The first and only technology platform capable of continuously and noninvasively measuring total hemoglobin (SpHb™) and oxygen content (SpOC™) (both pending FDA clearance), in addition to carboxyhemoglobin (SpCO™), methemoglobin (SpMet™), pleth variability index (PVI®), oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate—Masimo Rainbow SET is helping to advance patient safety and improve care worldwide.
"Our leading position in the world market is based on a history of implementing new technologies and innovative first-class solutions that create long-term value for our customers and their patients," said Schiller AG CEO, Alfred Schiller. "Masimo understands the importance of cutting-edge technologies in our market and has combined the most advanced noninvasive technology and capabilities with the best user benefits available into a technology platform that maximizes long-term value."
The ability to quickly and continuously measure SpHb, SpOC, SpMet, SpCO, PVI, SpO2, PR, and PI noninvasively may help clinicians to save lives by more rapidly diagnosing potentially life-threatening conditions, speeding treatment decisions and improving patient outcomes. SpHb may make hemoglobin testing quick, convenient and accessible to medical personnel in both acute and outpatient settings—enabling them to quickly identify conditions of anemia, or blood loss. The availability of real-time continuous SpOC may help to ensure optimal oxygen delivery in patients during rapidly-changing clinical situations.
By continuously and noninvasively monitoring SpMet, clinicians can accurately determine if drugs they are administering are causing methemoglobinemia, which can lead to brain damage and even death. Similarly, the ability to measure SpCO allows clinicians to detect elevated levels of carboxyhemoglobin that can be caused by everything from desiccated soda lime (known as "Monday Morning Phenomena"), or other poisons that can be introduced during surgery, to smokers reporting for surgery with high SpCO values. Increased SpCO compromises healing and may lead to death, while smokers with residual elevated COHb at the time of anesthesia are at cardiac ischemic risk. PVI provides clinicians with a continuous and noninvasive quantified measurement of changes in the perfusion index – indicating a change in a patient's physiologic condition, which may compromise cardiac function and affect systemic circulation – with potential clinical applications for noninvasive hypovolemia detection and fluid responsiveness monitoring.
"Establishing Masimo Rainbow SET as the technology engine for Schiller's patient monitoring, emergency medicine and rescue solutions enables us to provide a dynamic new set of clinical capabilities that expand the physiological data and options available to clinicians and caregivers – allowing them to better care for their patients," stated Dominik Doppler, VP, Marketing, Sales and Business Development at Schiller. "This is a compelling value proposition for the global healthcare market."
At the heart of Masimo Rainbow SET is the world's most accurate and reliable pulse oximetry technology – Masimo SET. More than 100 independent and objective studies demonstrate that Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. Before the introduction of Masimo SET in 1995, pulse oximetry was reliable only when patient conditions were ideal—on motionless patients with strong pulses and good perfusion. However, in the presence of patient motion, a weak pulse or low perfusion, excessive false alarms reduced the value of conventional pulse oximetry. Since then, Masimo SET Measure-Through Motion and Low Perfusion pulse oximetry has made continuous, noninvasive monitoring by pulse oximetry more reliable and clinically-relevant than ever before.
Joe E. Kiani, Chairman and CEO of Masimo, stated "Schiller has been a long-term partner with us and was one of the early-adopters of Masimo SET technology. Once again, as an early-adopter of Masimo Rainbow SET technology, Schiller represents a growing global community of Original Equipment Manufacturers (OEMs) who are embracing and standardizing to Masimo Rainbow SET as the noninvasive patient monitoring technology of the future. They are market leaders and global champions for innovation, patient safety and quality of care who make the world safer for patients everywhere. We appreciate the vision, leadership and commitment of Schiller and we are proud of our partnership."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™) and oxygen content (SpOC™) (both pending FDA clearance), carboxyhemoglobin (SpCO™), methemoglobin (SpMet™), and pleth variability index (PVI®), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that this agreement with Schiller and their adoption of Masimo technology will serve to substantially increase revenues, risks related to our assumption that Masimo's new noninvasive measurements—total hemoglobin (SpHb™) and oxygen content (SpOC™)—will deliver a sufficient level of clinical improvement over alternative hemoglobin testing capabilities to allow for rapid adoption of the technology and risks related to our assumptions regarding the timing or commercial availability of SpHb and SpOC, and will be timely cleared, if ever, by appropriate regulatory bodies, as well as other factors discussed in the "Risk Factors" section of our annual report on Form 10-K for the year ended December 29, 2007, filed with the Securities and Exchange Commission on March 4, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our annual report on Form 10-K for the year ended December 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


New Study Finds Masimo Rainbow SET Pulse CO-Oximetry™ Effective as a Universal Screening Tool to Identify Cases of Unsuspected CO Poisoning in ERs
Rhode Island Hospital study identifies 11 cases of unsuspected CO poisoning through universal CO screening with the Masimo Rad-57 Pulse CO-Oximeter™
Irvine, California—March 19, 2008 – Masimo, the inventor of Pulse CO-Oximetry and Measure-Through-Motion & Low Perfusion Pulse Oximetry, reported that a new clinical study, recently published in the Journal of Emergency Medicine, found the Masimo Rainbow SET Rad-57 Pulse CO-Oximeter to be "a safe, easily applied tool at triage that can identify cases of unsuspected elevated levels of carbon monoxide (CO) poisoning" that would otherwise have gone undetected.
Researchers at the Rhode Island Hospital, where the study was conducted, also concluded that universal SpCO® screening may prevent morbidity through early identification and treatment intervention, stating that: "we can point to several cases during our study period in which patient outcomes were different based upon availability of SpCO, recorded at triage."
The study titled "Noninvasive Pulse CO-Oximetry Screening in the Emergency Department Identifies Occult Carbon Monoxide Toxicity" was conducted over a nine-month period on more than 10,850 patients presenting to the Emergency Department at the Rhode Island Hospital in Providence, Rhode Island, by a research team of emergency medicine physicians from the Warren Alpert Medical School at Brown University and the Emergency Department of Rhode Island Hospital, headed by Dr. Selim Suner.
In this study, Dr. Suner, Dr. Jay, Dr. Partridge, Dr. Sucov, Dr. Valente, Dr. Chee and Dr. Hughes tested the ability to screen for CO toxicity in a busy tertiary center ED using the Masimo Rainbow SET Rad-57 Pulse CO-Oximeter and found 28 cases of CO toxicity (SpCO of > 9% for nonsmokers and >13% for smokers), of which 11 were unexpected, and were identified only with the aid of universal SpCO screening using the Masimo Rad-57. In all CO toxicity cases identified, venous or arterial COHb confirmations of elevated SpCO measurements were verified by lab analysis of blood samples taken with data results showing a "good correlation" between SpCO from the Masimo Rad-57 and COHb from the lab analysis.
The research team noted that identification of CO toxicity in the ED is often challenging because many patients may not know or suspect that they were exposed to CO and are unable to provide clinicians with sufficient history to prompt testing for carboxyhemoglobin (COHb). In addition, the symptoms of CO poisoning can be similar to the flu. However, missing the opportunity to diagnose CO poisoning at the ED because screening large populations of patients by invasive blood testing for CO toxicity is not practical and not routinely performed in the ED setting can lead to "inadvertently returning a patient to the site of CO exposure and may lead to further toxicity with the possibility of long-term neurological, psychiatric, or cardiovascular complications."
Using data extrapolated from the study at Rhode Island Hospital's level-1 trauma center ED, researchers suggest that potentially "as many as 11,000 occult poisoning cases" go undetected annually—illustrating the significant impact that universal SpCO screening could have on public health and safety. "Screening will also protect the public by identifying hidden sources of CO in households, workplaces and schools," said researchers.
Joe E. Kiani, Chairman and CEO of Masimo, stated: "The researchers at Rhode Island Hospital have done a great public service in addressing the benefits of routine, universal screening by Pulse CO-Oximetry upon admission in emergency departments. As this study has shown, the importance of diagnosing unsuspected CO poisoning at emergency departments nationwide could mean a big difference in the lives of the estimated 11,000 people each year who may be suffering in silence as CO ravages their health."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™) and oxygen content (SpOC™) (pending FDA clearance), carboxyhemoglobin (SpCO™), methemoglobin (SpMet™), and pleth variability index (PVI®), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that the Masimo Rainbow SET Rad-57 Pulse CO-Oximeter will be an effective universal screening tool at all EDs and risks related to our assumption that Masimo Pulse CO-Oximetry technology will deliver a sufficient level of clinical improvement over alternative carbon monoxide screening methods to allow for rapid adoption of the technology in hospital and emergency medicine environments, as well as other factors discussed in the "Risk Factors" section of our annual report on Form 10-K for the year ended December 29, 2007, filed with the Securities and Exchange Commission on March 4, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our annual report on Form 10-K for the year ended December 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Pulse CO-Oximetry and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


University of Michigan Hospitals and Health Centers Completes System-Wide Conversion to Masimo SET Pulse Oximetry Technology
Conversion helps U-M Hospitals and Health Centers, named one of America's best hospitals by U.S. News & World Report, make clinical practice changes to improve patient safety
Irvine, California – March 12, 2008 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, announced the completion of the University of Michigan (U-M) Hospitals and Health Centers system-wide implementation of Masimo SET pulse oximetry. Leaders at the institution cited superior clinical performance as the chief reason for conversion to Masimo SET as their standard of care for precise, continuous SpO2 monitoring.
Kevin K. Tremper, MD, Chairman of Anesthesiology at the U-M Hospitals and Health Centers, said, "Our evaluations of Masimo SET alongside other pulse oximetry technologies provided clinical staff with the opportunity to objectively appraise the performance of each of these pulse oximetry devices and we found the Masimo device to be superior."
Nationally recognized as one of the top healthcare organizations in the country and one of only 18 hospitals included in the U.S. News & World Report Best Hospitals Honor Roll, the U-M Hospitals and Health Centers is a 913-bed healthcare system encompassing three hospitals—University Hospital, C.S. Mott Children's Hospital and Women's Hospital—in addition to more than 30 health centers and 120 outpatient clinics.
Unlike conventional pulse oximeters, Masimo SET Measure-Through Motion and Low Perfusion pulse oximetry is new-generation technology that uses sophisticated signal processing technologies, including parallel engines and adaptive filters, to deliver accurate and reliable SpO2 and pulse rate measurements when conventional pulse oximetry technologies don't. As a result, Masimo SET provides the greatest sensitivity (ability to detect true positives) with the greatest specificity (ability to reject false positives). By delivering meaningful alarms and alerts that can be trusted to reflect a patient's true oxygenation status, clinicians can maximize their efficiency by concentrating on caring for their patients, rather than chasing false alarms.
The sensitivity and reliability of Masimo SET pulse oximetry technology can also provide an effective solution to address the clinical concerns associated with properly monitoring at-risk post-operative patients. Rising acuity levels—due predominately to aggressive post-operative pain management with patient-controlled analgesia (PCA) pumps and increased co-morbidities at admission—have dramatically increased incidents of avoidable adverse and sentinel events (events resulting in death or serious physical injury). In addition, undiagnosed Obstructive Sleep Apnea (OSA) can place patients at considerable respiratory risk as pain medications typically suppress the natural breathing reflex during apneic episodes. These clinical realities have significantly impacted the way in which clinicians deliver care.
"As a result of our new Masimo SET pulse oximetry capabilities, we have implemented a policy in which all patients receiving intravenous opioids for post-operative pain are monitored with Masimo SET oximeters networked to our nurse paging system," said Dr. Tremper. "We have found that this clinical practice change provides a workable solution to a challenging national clinical problem and, in addition to being well-received by our nursing staff, we feel it has improved patient safety within the U-M Health System. We are hoping to document and share our efforts in academic publications throughout the next year."
By making the conversion to Masimo, U-M Hospitals and Health Centers joins other top hospitals in the United States—including four of the top five, as listed on the U.S. News & World Report Honor Roll—that have all adopted Masimo SET as their primary pulse oximetry platform. More than 100 independent and objective studies demonstrate that Masimo SET provides the most trustworthy SpO2 readings even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. These studies have shown that Masimo SET delivers improvements in outcomes, safety and efficiency.
Joe E. Kiani, CEO of Masimo, stated, "University of Michigan Hospitals and Health Centers provides numerous local and statewide community health programs and services that enable healthcare access for all. This system-wide conversion and standardization to Masimo SET pulse oximetry is an example of U-M's commitment to advancing patient care and safety for all of its patients system-wide. We are delighted to be the pulse oximetry standard for U-M and their patients and are proud to provide a solution that supported their clinical practice change to help keep their post-operative patients monitored and safe."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™) and oxygen content (SpOC™) (pending FDA clearance), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and pleth variability index (PVI®), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that U-M's standardization to Masimo technologies will serve to provide substantially increased revenues for the company, risks related to our assumption that Masimo SET and Masimo Rainbow SET will deliver a sufficient level of clinical improvement over alternative pulse oximetry and patient monitoring systems to allow for rapid adoption of the technology, risks related to our assumption that Masimo SET pulse oximetry technology provides an effective solution to address the clinical concerns associated with properly monitoring at-risk post-operative patients and that this solution will lead to increased adoption of Masimo SET technology for monitoring such patients, and risks related to our assumptions regarding the timing or commercial availability of SpHb and SpOC, and will be timely cleared, if ever, by appropriate regulatory bodies, as well as other factors discussed in the "Risk Factors" section of our annual report on Form 10-K for the year ended December 29, 2007, filed with the Securities and Exchange Commission on March 4, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our annual report on Form 10-K for the year ended December 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, and Pulse CO-Oximeters are trademarks or registered trademarks of Masimo Corporation.


New Clinical Studies Presented at the 14th World Congress of Anesthesiology Demonstrate Masimo Advancements in Patient Care
Noninvasive Hemoglobin Receives Rave Reviews from Attendees at Masimo's Commercial Exhibit
Irvine, California – March 6, 2008 – Masimo, the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, reported that multiple clinical studies demonstrating the accuracy and clinical effectiveness of the Masimo Rainbow SET platform were highlighted earlier this week to over 8,000 anesthesiologists at the 14th World Congress of Anesthesiology (WCA) in Cape Town, South Africa. In addition, WCA attendees from all over the world were able to preview noninvasive total hemoglobin (SpHb™) and oxygen content (SpOC™) as part of the Rainbow SET platform (pending FDA clearance).
Clinical Study Highlights
Continuous Noninvasive Measurement of Hemoglobin via Pulse CO-Oximetry1, a clinical study led by Dr. Mark Macknet at Loma Linda University in Loma Linda, California, presented a study that compared an engineering prototype of Masimo Rainbow SET noninvasive total hemoglobin (SpHb) to invasive laboratory hemoglobin measurements in two groups. Group one included 55 patients scheduled to undergo surgery, while group two consisted of 32 healthy volunteers undergoing a hemodilution protocol. After reviewing 1,538 data pairs, researchers found that the Masimo technology accurately delivered total hemoglobin levels, with the study showing accuracy of 1.28 g/dl and 0.94 g/dl for group two, respectively, when compared to invasive laboratory CO-Oximetry. Researchers concluded that Masimo's device is the "first device developed that can continuously and noninvasively measure hemoglobin concentration, in addition to the other common hemoglobin species, and therefore provides a significant expansion of existing physiologic monitoring technology."
Casual Screening of Hemoglobin Noninvasively Positively Affects a Colleague's Future2, a case report by Dr. Martin Allard at Loma Linda University recounted the application of SpHb to assess an anemic hemoglobin level of 10.6 g/dl on a fellow anesthesiologist who otherwise appeared healthy. Invasive hemoglobin testing confirmed the measurement and further diagnostic testing revealed previously undiagnosed and asymptomatic esophageal cancer. Researchers concluded that Masimo SpHb allowed the "detection of this potentially devastating tumor before clinical signs or symptoms became apparent, which resulted in early intervention and therapy that may well be curative for this colleague."
New Pulse Oximetry Sensors with Low Saturation Accuracy Claims3, performed by Dr. Peter Cox at the Hospital for Sick Children in Toronto, Canada, evaluated 12 patients with congenital cyanotic cardiac lesions (CCCL) to compare noninvasive oxyhemoglobin (SpO2) measurements from the Masimo Rainbow SET Radical 7 device with Blue Sensor and the Covidien N-600 device (OxiMax with Lo-Sat) to invasive oxyhemoglobin levels from laboratory CO-Oximetry. Although the Nellcor N-600 with LoSat is advertised to work in CCCL patients, the accuracy demonstrated in this study was 6.49%, well outside of Nellcor's published specifications. In contrast, the Masimo Radical with Blue Sensor, the first and only sensor with accuracy claims cleared for cyanotic patients, performed within Masimo specifications and had significantly better accuracy at 3.85%. Study results demonstrate that the Masimo Blue sensor, which was "designed for use specifically in this patient population, is more accurate." Dr. Peter Cox, Hospital for Sick Children in Toronto, Canada, said, "accurate monitoring of oxygen saturations in children with cyanotic congenital heart defects is essential for appropriate patient management and, therefore, its impact on their long-term outcome. The Masimo Blue Sensor accurately tracks saturation to levels as low as 60%, which will greatly assist caregivers in the management of this patient population."
Severe Methemoglobinemia Detected by Pulse CO-Oximetry in the Operating Room4, a case report by Dr. Steven J. Barker and Dr. E. H. Annabi at the University of Arizona in Tucson, Arizona, documented the use of Masimo noninvasive methemoglobin (SpMet®) to accurately diagnose a severe case of drug-induced methemoglobinemia and subsequently monitor and guide the patient's treatment and recovery. Researchers concluded that Masimo SpMet can "quickly diagnose" methemoglobinemia in the perioperative setting, where time is of the utmost essence.
Commercial Exhibit Highlights
Masimo also previewed, for the first time, continuous noninvasive total hemoglobin (SpHb) and oxygen content (SpOC) as part of the Rainbow SET platform during WCA's commercial exhibition. In the first five hours alone, an astounding number of over 1,000 anesthesiologists visited the booth and experienced first-hand product demonstrations and clinical presentations of the new SpHb and SpOC parameters, along with Masimo's measure-through motion and low perfusion pulse oximeters. Scores of anesthesiologists who perform invasive hemoglobin testing routinely during surgery were amazed by the ability to get their own hemoglobin levels tested noninvasively in just seconds. In fact, anesthesiologists were heard proclaiming "this changes everything" and "noninvasive hemoglobin will revolutionize anesthesiology!"
"The new clinical evidence for Masimo Rainbow SET and preview of SpHb and SpOC were extremely well-received by anesthesiologists from around the world at the WCA," stated Joe E. Kiani, Founder and CEO of Masimo. "We are proud to once again revolutionize noninvasive monitoring for the benefit of patient care."
Michael O'Reilly, MD, EVP of Medical Affairs at Masimo, stated, "Noninvasive total hemoglobin represents an exciting and valuable expansion of the noninvasive hemodynamic capabilities available to anesthesiologists. Many of whom remarked that the ability to see total hemoglobin and oxygen content measurements, along with carboxyhemoglobin (SpCO®), methemoglobin (SpMet), pleth variability index (PVI®), perfusion index (PI), oxygen saturation (SpO2) and pulse rate—all on one screen, with one device and one sensor, was equally impressive."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and pleth variability index (PVI®), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that Masimo's new noninvasive measurements—total hemoglobin (SpHb™) and oxygen content (SpOC™)—will deliver a sufficient level of clinical improvement over alternative hemoglobin testing capabilities to allow for rapid adoption of the technology and risks related to our assumptions regarding the timing or commercial availability of SpHb and SpOC, and will be timely cleared, if ever, by appropriate regulatory bodies, as well as other factors discussed in the "Risk Factors" section of our annual report on Form 10-K for the year ended December 29, 2007, filed with the Securities and Exchange Commission on March 4, 2008. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our annual report on Form 10-K for the year ended December 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
1 Continuous Noninvasive Measurement of Hemoglobin via Pulse CO-Oximetry. Mark R. Macknet, Penny L. Kimball-Jones, Richard L. Applegate, Robert D. Martin, Martin W. Allard. Anesthesiology, Loma Linda University, Loma Linda, CA.
2 Casual Screening of Hemoglobin Noninvasively Positively Affects a Colleague's Future. Martin Allard, John Viljoen, Mark Macknet. Anesthesiology, Loma Linda University, Loma Linda, CA.
3 New Pulse Oximetry Sensors with Low Saturation Accuracy Claims. Peter Cox. Department of Critical Care Medicine, Hospital for Sick Children, Toronto, Canada.
4 Severe Methemoglobinemia Detected by Pulse CO-Oximetry in the Operating Room. S. J. Barker, E.H. Annabi. Anesthesiology, University of Arizona, Tucson, AZ.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, and Pulse CO-Oximeters are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports Fourth Quarter 2007 and Full Year 2007 Financial Results
Record results mark 18 th consecutive quarter of revenue growth
2007 Highlights:
- Product revenues increased 29% to a record $199.7 million
- Masimo SET pulse oximeter units increase 20% to 116,300 units
Irvine, California, February 26, 2008 – Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion & Low Perfusion pulse oximetry, today announced its financial results for both the quarter and year ended December 29, 2007.
For the fiscal fourth quarter, Masimo reported product revenues of $55.2 million representing a 29% increase over $42.9 million for the fourth quarter of 2006. Including royalty revenues, Masimo reported total fourth quarter revenues of $69.3 million compared to $61.6 million for the fourth quarter of 2006. Net income for the quarter was $12.1 million representing $0.20 earnings per common share, including $0.02 per common share relating to a year-to-date tax benefit recorded in the fourth quarter. Masimo also reported that it shipped 29,400 Masimo SET and Masimo Rainbow SET oximetry units, excluding handheld units, during the fourth quarter of 2007, up 10% from 26,700 in the comparable prior year period, resulting in a new estimated worldwide installed base of 470,000 Masimo SET pulse oximeters.
For the year ended December 29, 2007, Masimo's product revenues were $199.7 million, up 29% from $155.1 million in 2006. Including royalty revenues, Masimo's total revenues were $256.3 million for the year ended December 29, 2007, up from $224.3 million in 2006. In the year ended December 29, 2007, Masimo shipped 116,300 Masimo SET and Masimo Rainbow SET pulse oximeter units, excluding handheld pulse oximeters, compared to 96,600 in 2006, representing a 20% increase in new pulse oximeter and Pulse CO-Oximeter shipments.
Net income for the year ended December 29, 2007, was $42.3 million compared to $181.8 million in 2006, which included $262.6 million in net patent litigation settlement proceeds and various one-time stock option based bonus payments related to a January 2006 patent litigation settlement. For the year ended December 29, 2007, Masimo's reported net income attributable to common stockholders was $23.1 million, or $0.60 per common share, as compared to $3.04 per common share for the year ended December 31, 2006.
Cash, cash equivalents and short-term investments rose to $96.7 million at December 29, 2007 up from $88.6 million at September 29, 2007 and from $55.4 million at December 31, 2006.
Financial Guidance
For the full year 2008, Masimo expects total revenues to be approximately $292 million and total product revenues to be approximately $246 million. Masimo also expects full year 2008 earnings per common share to be approximately $0.52 per share. Included in the $0.52 per common share projection is approximately $11.0 million in expected 2008 non cash stock based compensation charges, up from $3.9 million in 2007. Stock based compensation charges are expected to increase due principally to the increase in the market price of our common stock and to due to the increase in the number of options granted consistent with the increase in our total employee headcount. The projections and guidance set forth above are estimates only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 2:00 p.m. PT (5:00 p.m. ET) to discuss the results. The dial-in numbers are (800) 295-4740 for domestic callers and (617) 614-3925 for international callers. The reservation number for both dial-in numbers is 39491099. A live webcast of the conference call will be available online from the "investor relations" page of the Company's corporate website at www.masimo.com.
After the live webcast, the call will remain available on Masimo's website through March 26, 2008. In addition, a telephonic replay of the call will be available until March 10, 2008. The replay dial-in numbers are (888) 286-8010 for domestic callers and (617) 801-6888 for international callers. Please use reservation code 89834871.
The financials results included in this release are unaudited. The complete audited financial statements of the company for the year ended December 29, 2007 will be included in the Masimo Annual Report on Form 10-K, to be filed with the SEC early next month.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the Company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), pending regulatory approval, carboxyhemoglobin (SpCO™) and methemoglobin (SpMet™), pleth variability index (PVI®), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com
Forward-Looking Statements
This press release includes forward-looking statements. All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our plans, objectives and prospects regarding, among other things, our financial condition, results of operations, prospects and business generally; the market acceptance of our technologies and products ; the value of measuring new parameters; expectations regarding our ability to design and deliver innovative new noninvasive technologies, such as the recently introduced total hemoglobin measurement, and our assumption of total hemoglobin's timely regulatory clearing by appropriate regulatory bodies, if ever, and our assumption to expand into additional areas of vital signs monitoring and measurements; and expectations for total revenues, product revenues, GAAP earnings per share, non-GAAP earnings per share and stock based compensation expenses for the full fiscal year 2008. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our reliance on Masimo SET and related products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; the failure to continue developing innovative products and technologies; the introduction of competing products; the lack of acceptance of any new products and technologies, including the recently announced total hemoglobin measurement and including whether regulatory clearances will be obtained, the loss of our customers; increases in prices for raw materials or the loss of key supplier contracts; the failure to retain and recruit senior management and manage expected growth; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended September 29, 2007, filed with the Securities and Exchange Commission on November 1, 2007. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended September 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Masimo Corporation
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
949-297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpCO, SpMet, PVI and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
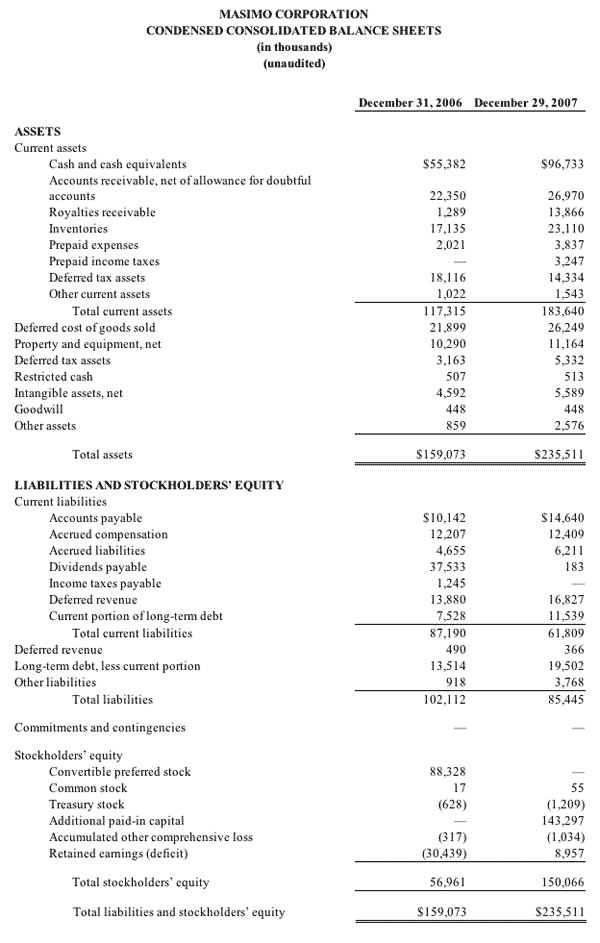
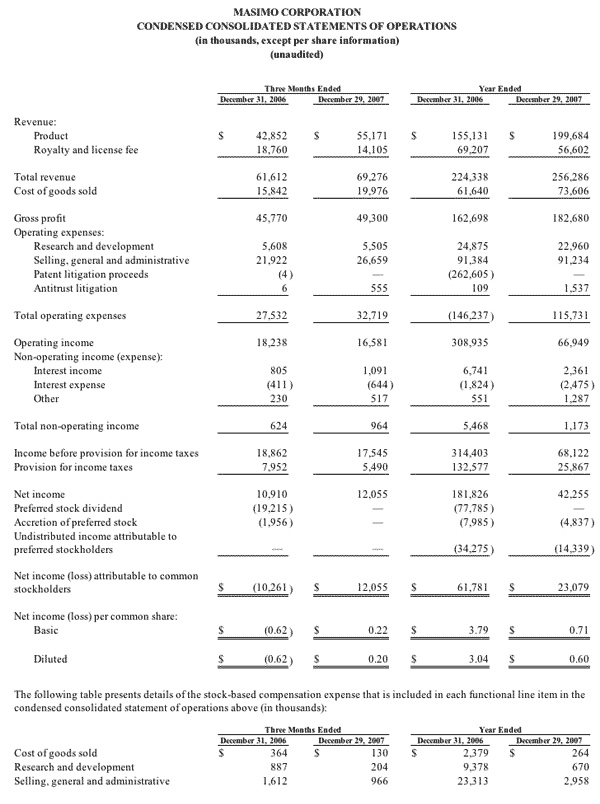
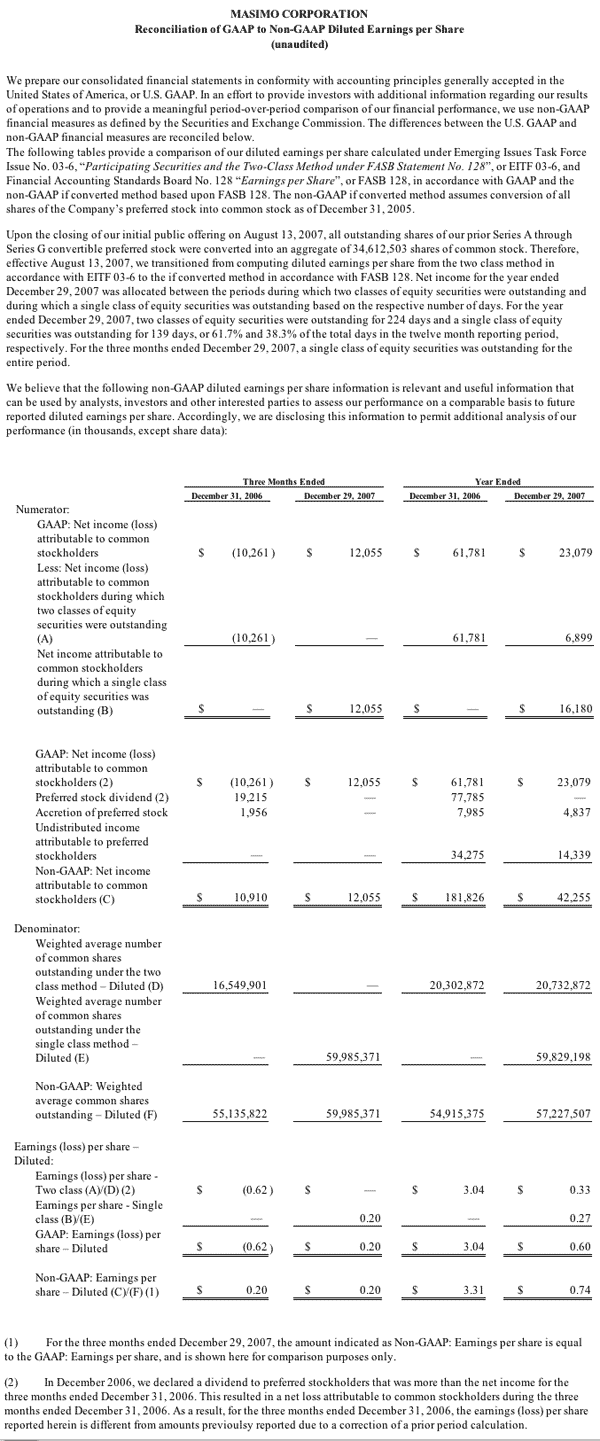


Masimo Announces Continuous Noninvasive Total Hemoglobin
Masimo to debut this latest Rainbow SET® parameter at the upcoming World Congress of Anesthesiologists Meeting
Irvine, California - February 25, 2008 - Masimo, the inventor of Pulse CO-Oximetry™ and Measure-Through Motion and Low Perfusion pulse oximetry, announced that it will debut its breakthrough technology for noninvasive and continuous total hemoglobin (SpHb™) and oxygen content (SpOC™) monitoring at the World Congress of Anesthesiology (WCA) in Cape Town, South Africa, on March 3, 2008.
The advent of noninvasive total hemoglobin within the Masimo Rainbow SET platform will make hemoglobin testing more convenient and broadly available to medical personnel in both the acute and outpatient settings—the measurement is instantaneous and pain-free. Over 350 million hemoglobin tests are done in the US alone each year. Prior to Masimo Rainbow SET, invasive and time-consuming lab tests were the only methods available to determine total hemoglobin levels which provided delayed and intermittent data.
Masimo expects to make SpHb and SpOC shipments to select customers for clinical use in the second half of 2008, pending regulatory clearances. There is a 510(k) pending for SpHb and SpOC in the US.
Martin Allard, M.B.Ch.B, FRC, Professor and Director of Research, Department of Anesthesiology, Loma Linda University, stated, "From its application as a noninvasive tool for universal hemoglobin screening at routine health check-ups to the management of blood loss and blood replacement in patients experiencing acute blood loss, or those undergoing surgery—these new measurements could prove to be indispensable in a wide variety of healthcare settings."
"Noninvasive, continuous total hemoglobin and oxygen content monitoring should allow for more timely interventions both inside and outside of the operating room and should enable emergency medical professionals, dialysis centers, family physicians, cardiologists and other care providers to better care for their patients," said Joe E. Kiani, Founder & CEO of Masimo.
For the first time, the Masimo Rainbow SET technology platform will provide clinicians with access to real-time trending and tracking of a patient's total hemoglobin status enabling them to quickly identify conditions of anemia, or blood loss. When patients undergo blood transfusions, clinicians will be able to use Masimo Rainbow SET SpHb to titrate blood and maintain hemoglobin levels within acceptable ranges. Additionally, continuous monitoring of hemoglobin levels may provide clinicians with an early warning of possible internal hemorrhaging in the emergency department, trauma and post-op settings. Because blood and hemoglobin restoration must be carefully titrated to targeted levels to avoid potentially serious morbidities, Masimo Rainbow SET SpHb provides instant feedback that the proper levels are achieved, advancing patient safety and accelerating recovery. On July 30, 2007, The Centers for Medicare and Medicaid Services released their final National Coverage Determination (NCD) restricting coverage for the treatment of anemia, specifically Erythropoiesis Stimulating Agents (ESA) therapy, to when the hemoglobin level is less than 10g/dL. This ruling makes hemoglobin testing a prerequisite to coverage and possibly administration of ESA therapy in certain patients.
"I believe the availability of Masimo Rainbow SET SpHb will revolutionize the management of anemia across clinical settings and scenarios and could provide a solution for those looking to satisfy The Centers for Medicare and Medicaid Services' new rules for hemoglobin testing," stated Michael O'Reilly, MD, EVP of Medical Affairs at Masimo.
A simple upgrade to most Masimo Radical pulse oximeters is all that will be necessary to transform an existing monitor to Masimo Rainbow SET performance - enabling integration of noninvasive total hemoglobin monitoring into any clinical setting.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures total hemoglobin (SpHb™), oxygen content (SpOC™), carboxyhemoglobin (SpCO™), methemoglobin (SpMet™), and pleth variability index (PVI®), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that Masimo's new noninvasive measurements—total hemoglobin (SpHb™) and oxygen content (SpOC™)—will deliver a sufficient level of clinical improvement over alternative hemoglobin testing capabilities to allow for rapid adoption of the technology and risks related to our assumptions regarding the timing or commercial availability of SpHb and SpOC, and will be timely cleared, if ever, by appropriate regulatory bodies, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended September 29, 2007, filed with the Securities and Exchange Commission on November 1, 2007. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended September 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, and Pulse CO-Oximeters are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report Fourth Quarter and Fiscal Year 2007 Financial Results on February 26, 2008
Conference call and webcast with Masimo to begin at 2:00 pm PT (5:00 pm ET)
IRVINE, Calif., February 19, 2008—Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, today announced it will release fourth quarter and fiscal year 2007 financial results for the period ended December 29, 2007, after the market closes on Tuesday, February 26, 2008.
A conference call to review the results will begin at 2:00 p.m. PT (5:00 p.m. ET) and will be hosted by Joe E. Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President & Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the Company's corporate website at www.masimo.com. The dial-in numbers are (800) 295-4740 for domestic callers and (617) 614-3925 for international callers. The reservation number for both dial-in numbers is 39491099. After the live webcast, the call will remain available on Masimo's website through March 26, 2008. In addition, a telephonic replay of the call will be available until March 10, 2008. The replay dial-in numbers are (888) 286-8010 for domestic callers and (617) 801-6888 for international callers. Please use reservation code 89834871.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures carboxyhemoglobin (SpCO™) and methemoglobin (SpMet™), pleth variability index (PVI®), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
Masimo Corporation
(949) 297-7080
mderaad@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
949-297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, PVI and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


National Fire Protection Association (NFPA) Sets New National Standard for CO Screening by Pulse CO-Oximetry™
2008 NFPA 1584 establishes the routine use of Pulse CO-Oximetry as a way to protect the lives of the nation's firefighters from the dangers of CO Poisoning
Irvine, California - February 19, 2008 - Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, announced today that the National Fire Protection Association (NFPA) has included Carbon Monoxide (CO) screening by Pulse CO-Oximetry as part of a new national healthcare standard for firefighters potentially exposed to Carbon Monoxide poisoning. NFPA's consensus codes and standards serve as the worldwide authoritative source on fire prevention and public safety—virtually every building, process, service, design, and installation in society today is affected by NFPA documents.
The new standard, which became effective December 31, 2007 and was recently published, establishes that "any firefighter exposed to CO or presenting with headache, nausea, shortness of breath, or gastrointestinal symptoms" should be measured for CO poisoning by Pulse CO-Oximetry or other approved methods. It also requires every fire department to establish Standard Operating Guidelines (SOGs) that outline uniform rehabilitation procedures for firefighters at incident scenes and training exercises.
Too often, even the most skilled first responders miss the chance to treat carbon monoxide poisoning early because, until Masimo invented Masimo Rainbow SET Pulse CO-Oximetry in 2005, there wasn't a noninvasive way to detect elevated levels of CO in the blood. With the Masimo Rad-57 Pulse CO-Oximeter, fire fighters, EMS professionals and ER clinicians can easily detect carbon monoxide poisoning by applying a noninvasive LED-based sensor on the victims or themselves, allowing for prompt and possibly life-saving treatment that can also limit the likelihood of long-tern cardiac and neurological damage.
Studies have shown that even a single high level exposure, or prolonged exposure to low levels of CO, has the potential to cause long-term heart, brain and organ damage. Long-term effects of CO include: cardiac arrests, Parkinson-like syndromes affecting motor skills and speech, dementia, cortical blindness, acute renal failure, and muscle cell death.
"Often cited by attorneys within the legal system, NFPA standards represent complete industry consensus and are supported by a substantial amount of scientific or medical evidence," said Mike McEvoy, EMS Director, Board of New York State Association of Fire Chiefs. "This new national standard adds considerable weight to growing industry guidance calling for CO screening by leading EMS, EMT and firefighter associations nationwide, including the National Association of Emergency Medical Technicians (NAEMT), the International Association of Firefighters (IAFF), and the National Association of EMS Educators (NAEMSE)."
Joe E. Kiani, Chairman and CEO of Masimo, stated "We applaud NFPA for making CO screening for firefighters a national standard with this latest revision of NFPA 1584 and for taking the lead in healthcare reform for all of North America's firefighters. Establishing uniform standards is crucial to ensuring that the nation's firefighters receive the proper care and attention required to help keep them safe, healthy and in peak condition to be able to meet the demands of their life-saving work. We are proud that our Pulse CO-Oximetry technology can play such a vital role within this standard and in the lives of our heroic public servants."
A worldwide leader in providing fire, electrical, building, and life safety to the public since 1896, NFPA's mission is to reduce the global burden of fire and other hazards on the quality of life by providing and advocating consensus codes and standards, research, training, and education. NFPA's 300 codes and standards influence every building, process, service, design, and installation in the U.S. and many other countries. With a membership of more than 81,000 and over 80 national trade and professional organizations, NFPA is the authority on fire, electrical, and building safety. Copies of the National Fire Protection Association (NFPA) Section 1584, Standard on the Rehabilitation Process for Members During Emergency Operations and Training Exercises, are now available through the NFPA.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate that Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures Carboxyhemoglobin (SpCO™) and Methemoglobin (SpMet™), Pleth Variability Index (PVI®), in addition to Oxyhemoglobin (SpO2), Perfusion Index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that inclusion in the new 2008 NFPA 1584 as a national standard will serve to substantially increase sales or revenues for the company and risks related to our assumption that the Masimo Rad-57 Pulse CO-Oximeter will deliver a sufficient level of clinical improvement over alternative CO monitoring devices to allow for rapid adoption of the technology at hospitals, fire and rescue, EMT and EMS units, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended September 29, 2007, filed with the Securities and Exchange Commission on November 1, 2007. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended September 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, PVI and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


The University of California at San Francisco Medical Center Pioneers System-wide Adoption of Masimo Rainbow SET Technology
One of the nation's top five hospitals, UCSF converts to Masimo SET pulse oximetry and leverages revolutionary new noninvasive measurement capabilities
Irvine, California-February 13, 2008 - Masimo, the inventor of Pulse CO-Oximetry and Measure-Through-Motion & Low Perfusion Pulse Oximetry, today announced the completion of UCSF Medical Center's system-wide implementation of Masimo SET pulse oximetry and the Masimo Rainbow SET technology platform-establishing UCSF Medical Center as the first hospital to implement Masimo Rainbow SET capabilities system-wide.
Initially, UCSF Medical Center performed an extensive pulse oximetry comparison and thorough evaluation spanning virtually every department and found that Masimo SET's obtained accurate and reliable oxygen saturation measurements under difficult conditions, including patient motion and low perfusion, as the chief reason for conversion to Masimo SET. Also, as part of the evaluation, UCSF clinicians utilized new Masimo Rainbow SET technology to noninvasively and continuously measure physiologic and hemodynamic components that were previously only available by invasive tests. As a result, UCSF decided to expand the adoption of Masimo technologies beyond pulse oximetry to include the noninvasive patient monitoring capabilities of the Masimo Rainbow SET technology platform.
UCSF Medical Center, a 600-bed hospital and academic medical center, is among the top five hospitals in the nation according to U.S. News and World Report. UCSF Medical Center, which also includes the 180-bed UCSF Children's Hospital, Mount Zion for outpatient care and specialized programs, and more than 60 outreach clinics, is also home to the only nationally-designated Comprehensive Cancer Center and the only nationally designated Center of Excellence in Women's Health in Northern California.
"Previously, low perfusion and patient motion were two of our toughest challenges and the biggest obstacles to obtaining the accurate and reliable SpO2 measurements we rely on to guide our treatment decisions," said Patricia Roth, MD, Medical Director of Anesthesia Operating Room Support Services, Associate Professor of Anesthesia & Perioperative Care, UCSF Medical Center, San Francisco, California. "Masimo SET provides continuous, accurate oxygen saturation measurements that reflect a patient's true status-even during low perfusion and motion. In addition, Masimo adhesive sensors have proven to be more robust, allowing us to use just one sensor from Pre-Op, OR (Operating Room) and PACU (Post-Anesthesia Care Unit) through to the general care floor or ICU (Intensive Care Unit), while Masimo SET Perfusion Index (PI) and Pleth Variability Index (PVI) measurements have exciting potential clinical applications and added value."
In addition to their system-wide standardization on Masimo SET pulse oximetry, UCSF also wanted to ensure that every site within their system was Rainbow-enabled-allowing UCSF clinicians to utilize advanced Masimo Rainbow SET technology and revolutionary new noninvasive measurement capabilities throughout their network facilities. Masimo Rainbow SET is an upgradable noninvasive blood constituent monitoring platform capable of measuring blood constituents that previously required invasive procedures. Masimo Rainbow SET's first application is Pulse CO-Oximetry, the first and only technology platform capable of continuously and noninvasively measuring Carboxyhemoglobin (SpCO™) and Methemoglobin (SpMet™), in addition to Oxyhemoglobin (SpO2), Perfusion Index (PI), Pleth Variability Index (PVI®) and pulse rate.
By quickly and accurately determining levels of SpCO and SpMet-two critical dyshemoglobins proven to increase morbidity and mortality in a broad range of clinical settings-clinicians can accurately determine their patients' true oxygenation status. PVI provides a continuous and noninvasive quantified measurement of changes in the perfusion index that may compromise cardiac function and affect systemic circulation, with potential clinical applications for noninvasive hypovolemia detection and fluid responsiveness monitoring. These new noninvasive measurement capabilities, unique to the Masimo Rainbow SET technology platform, allow for more precise and timely diagnosis and treatment.
Joe E. Kiani, CEO and Chairman of Masimo, stated; "UCSF Medical Center is recognized throughout the world for offering pioneering treatments. From becoming the first in the world to successfully perform surgery on a baby still in the womb to developing life-saving treatments for premature infants whose lungs aren't fully developed, UCSF is now pioneering the application of new noninvasive measurements enabled by the Masimo Rainbow SET technology platform to help advance the care they deliver now and into the future. This combination of innovative medicine, advanced technology and compassionate care is what makes UCSF a world leader in healthcare. We are proud of our pioneering partnership with UCSF."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate that Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures Carboxyhemoglobin (SpCO™) and Methemoglobin (SpMet™), Pleth Variability Index (PVI®), in addition to Oxyhemoglobin (SpO2), Perfusion Index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that UCSF Medical Center's standardization to Masimo technologies will serve to provide substantially increased revenues for the company and risks related to our assumption that Masimo SET and Masimo Rainbow SET will deliver a sufficient level of clinical improvement over alternative pulse oximetry and patient monitoring systems to allow for rapid adoption of the technology, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended September 29, 2007, filed with the Securities and Exchange Commission on November 1, 2007. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended September 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Dana Banks
Masimo Corporation
949-297-7348
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, PVI and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Honored with FDNY 'Flag of Heroes'
Recently, Masimo was recognized with the New York Fire Department's (FDNY) highest honor - the 'Flag of Heroes' - in appreciation of the company's tremendous contribution to the lifesaving efforts of the FDNY and the greater public health in the battle against the 'silent killer' known as carbon monoxide (CO) poisoning.
The 'Flag of Heroes' is one of only 3,000 U.S. flags that was previously flown at half mast on the first anniversary of the terrorist attacks at the World Trade Center on September 11, 2001. The limited-edition flag contains the names of 343 brave FDNY men and women and emergency services personnel who gave their lives in service, to save others on 9/11.
Joe E. Kiani, Chairman and CEO of Masimo stated; "Since we first debuted our invention of Masimo SET in 1995, we have received dozens of honors, but none have touched me more than this one. The 'Flag of Heroes' is a compelling symbol of the brave and unselfish contribution of 343 of FDNY's finest lifesavers. During the presentation, representatives of the FDNY EMS Command told us that they have already saved numerous lives with our invention and count us among their heroes because we have provided them with a product that not only saves the lives of their patients, but is also saving the lives of firefighters and rescuers."

The FDNY 'Flag of Heroes' was presented to Masimo CEO Joe Kiani by FDNY EMS Lt. James Fallar (L) and Paramedic Joe Kudak (R) at Masimo's International Sales Meeting in Dana Point, California, on January 25, 2008.

Unveiling the 'Flag of Heroes'
FDNY Paramedic Joe Hudack and Mike McEvoy, board member of the New York State Association of Fire Chiefs.
A Flag of Heroes for Heroes
"On behalf of all of us at Masimo, who work tirelessly to do what's right for patient care by innovating and elevating healthcare to a new level, I deeply, respectfully and sincerely thank FDNY for recognizing the hero in us all."
Joe Kiani
Chairman and CEO
Masimo



National Association of Emergency Medical Technicians (NAEMT) Supports Prehospital Screening for Carbon Monoxide in the Blood
Association heightens awareness for significant public health hazard of CO poisoning and advises screening of CO levels in the blood as a way to meet the challenge and improve the quality of care
Irvine, California - January 29, 2008 - Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Measure-Through Motion and Low Perfusion pulse oximetry, today announced that the National Association of Emergency Medical Technicians (NAEMT) supports the use of routine field screening protocols for the detection of elevated carbon monoxide (CO) levels in the blood of any patient presenting with suspected exposure or symptoms.
In a letter to its members and EMS professionals this month, NAEMT highlighted that "failure to diagnose may lead to improper treatment and transport decisions for victims of carbon monoxide poisoning" and recommended proper CO training, along with noninvasive detection protocols for the recognition and management of carbon monoxide poisoning, by all field EMS personnel as a way to improve patient care and protect the public from the "significant public health hazard" of carbon monoxide. The introduction of four new CO training programs, available free to NAEMT members online helps the association build awareness and promote adequate protocols for addressing this public health challenge.
NAEMT joins other industry-leading emergency first responder associations, including the National Association of EMS Educators (NAEMSE) and the International Association of Fire Fighters (IAFF), who have recently issued similar recommendations that EMS and fire professionals "noninvasively screen patients for carbon monoxide poisoning that have had a suspected exposure, or present with any of the signs or symptoms of carbon monoxide poisoning." These organizations are examples of a growing trend within the emergency services industry and the convergence toward a new standard of care for the proactive screening of CO-exposed patients and emergency services personnel by newly developed Pulse CO-Oximetry™ technology.
NAEMT President Jerry Johnston said "The new training programs are designed to close the knowledge gap between carbon monoxide poisoning and available noninvasive respiratory gas monitoring tools, like Pulse CO-Oximetry, for both EMTs and paramedics. We believe that Pulse CO-Oximetry represents a vital component in the rapid, noninvasive detection of CO levels in the blood of patients at the scene of emergencies, where critical diagnosis and treatment decisions are initiated and most effective."
Too often, even the most skilled first responders can miss the chance to treat carbon monoxide poisoning early because until now there hasn't been a fast, accurate and noninvasive way to detect elevated levels of CO in the blood. However, with the Masimo Rainbow SET Rad-57 Pulse CO-Oximeter-the first and only technology capable of continuously and noninvasively measuring carbon monoxide levels in the blood-EMS professionals can easily detect carbon monoxide poisoning on the spot in just seconds with the push of a button, allowing for prompt and possibly life-saving treatment. In addition, the Masimo Rad-57 can also limit the likelihood of long-term cardiac and neurological damage that can result from non-fatal exposures.
Studies have shown that even a single high level exposure, or prolonged exposure to low levels of CO, has the potential to cause long-term cardiac, neurocognitive and psychiatric damage. The long-term effects of CO-including Parkinson-like syndromes affecting motor skills and speech, dementia, cortical blindness, acute renal failure, muscle cell death, and more-can often be nearly as devastating for victims and their families as its mortality.
NAEMT is the nation's largest and oldest organization solely representing the professional interests of more than 34,000 paid and volunteer EMS workers from across the United States and 57 foreign countries who provide on-the-scene emergency care to populations around the world.
Joe E. Kiani, Chairman and CEO of Masimo, stated "NAEMT recommendations specifically address the importance and necessity of screening for CO levels in the blood on the scene of an emergency, where appropriate recognition and management of CO poisoning can make a life-saving difference. We invented Masimo Rainbow SET Pulse CO-Oximetry to provide clinicians with a noninvasive way to quickly, easily and accurately measure CO levels in a patients' and rescuers' blood anywhere and anytime. The implication of our unique technology for the advancement of public safety initiatives worldwide by industry-leading organizations like NAEMT is an important step to eliminating unnecessary deaths and long-term health consequences associated with CO poisoning."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate that Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures carboxyhemoglobin (SpCO), methemoglobin (SpMet), and pleth variability index (PVI), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that the Masimo Rad-57 Pulse CO-Oximeter will deliver a sufficient level of clinical improvement over alternative CO monitoring devices to allow for rapid adoption of the technology at hospitals, fire and rescue, EMT and EMS units, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended September 29, 2007, filed with the Securities and Exchange Commission on November 1, 2007. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended September 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, PVI and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Awarded Multi-Year Pulse Oximetry Agreement with HealthTrust Purchasing Group
Masimo technologies now available through the nation's top-10 GPOs1
Irvine, California, January 21, 2008 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced it has signed a three-year supplier agreement with HealthTrust Purchasing Group (HealthTrust), representing more than 3,800 facilities, including acute care hospitals, ambulatory surgery centers, alternate care sites and physician practices, with an annual purchasing volume of more than $13 billion. The agreement provides HealthTrust members access to Masimo SET pulse oximetry and Masimo Rainbow SET monitoring technologies - enabling increased patient safety and improved quality of care.
The addition of HealthTrust expands Masimo's GPO contracts to include all 10 of the nation's top GPOs, allowing hospitals and other care providers increased access to Masimo SET and Masimo Rainbow SET technologies. This agreement makes Masimo technologies, bedside and handheld pulse oximetry monitors and Patient Safety Net available to over 3,800 HealthTrust acute member facilities nationwide.
More than 100 independent and objective studies demonstrate that Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. Masimo Rainbow SET is a breakthrough noninvasive blood constituent monitoring platform that with a single noninvasive sensor can measure many blood constituents that previously required invasive procedures. Masimo Rainbow SET's first application is Pulse CO-Oximetry, the first and only technology platform capable of continuously and noninvasively measuring carboxyhemoglobin (SpCO™) and methemoglobin (SpMet™) levels in the blood, pleth variability index (PVI), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate.
Masimo has an unparalleled breadth and depth of pulse oximeter and sensor solutions as well as its proprietary SatShare interface, which allows a simple upgrade of an existing monitor to Masimo SET performance. And since Masimo SET is the leading Read-Through Motion and Low Perfusion SpO2 solution in more than 100 multiparameter monitors and 50 monitoring brands, hospitals can easily integrate Masimo technology into any clinical setting, enabling efficient whole-house conversions.
About HealthTrust
HealthTrust Purchasing Group, headquartered in Brentwood, Tennessee, is a group purchasing organization that supports over 3,800 not-for-profit and for-profit facilities including acute care hospitals, ambulatory surgery centers, physician practices, and alternate care sites. With an annual purchasing volume by its members of more than $13 billion, HealthTrust is committed to obtaining the best price for clinically-recommended products, ensuring their timely delivery and continuously evaluating and improving its services to the patients, physicians and clinicians it serves. HealthTrust is located at 155 Franklin Road, Suite 400, Brentwood, TN 37027 Website: www.healthtrustpg.com.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate that Masimo SET provides the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures carboxyhemoglobin (SpCO), methemoglobin (SpMet), and pleth variability index (PVI), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that entering into a multi-year agreement with HealthTrust will serve to provide substantially increased revenues for the company, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended September 29, 2007, filed with the Securities and Exchange Commission on November 1, 2007. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended September 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
1 Healthcare Purchasing News research, May 2006 (www.hpnonline.com/resources/GPOs.html)
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications,Patient SafetyNet, Rainbow, SpCO, SpMet, PVI and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Abington Memorial Hospital Completes System-Wide Conversion to Masimo SET Pulse Oximetry Technology
Teaching hospital sets the pace for healthcare in the Philadelphia suburbs with unique new capabilities enabled by Masimo SET and Masimo Rainbow SET technologies
Irvine, California - January 11, 2008 - Abington Memorial Hospital and Masimo (NASDAQ:MASI), the inventor of Pulse CO-Oximetry and Read-Through-Motion & Low Perfusion Pulse Oximetry, have announced the completion of Philadelphia-based Abington Memorial Hospital's system-wide conversion to Masimo SET Pulse Oximetry. The hospital performed an extensive and thorough evaluation of Masimo SET technology and cited clinical preference, patient safety and unique capabilities as key factors for the conversion.
Dr. Ara Moomjian, Chief of Neonatology at Abington Memorial Hospital, said "When surveyed, Abington clinicians clearly preferred Masimo SET technology over other available pulse oximetry solutions and the superior performance of Masimo SET during multi-departmental evaluations only served to cement the decision."
Abington Memorial Hospital is a 570-bed regional teaching hospital in Abington, Pennsylvania. With more than 34,000 inpatient admissions and greater than 5,000 births annually, the hospital is a major regional referral center for cancer care, cardiac care, and surgery (including orthopaeadic surgery and neurosurgery) and maintains the only Level II trauma center in Montgomery County.
By making the conversion to Masimo, Abington Memorial Hospital joins other top hospitals in the United States-including four of the top five-as listed on the US News & World Report Honor Roll, which have all adopted Masimo SET as their primary pulse oximetry platform. Masimo SET is clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 readings even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. These studies demonstrate that Masimo SET delivers improvements in outcomes, safety and efficiency.
"After converting to Masimo SET, we noticed a significant decrease in false alarms," said Dr. Stephen Snyder, Neonatologist at Abington Memorial Hospital. "In the past, other monitors would falsely alarm in the presence of motion or low perfusion, which would prolong the patients' length of stay. Hospital protocols dictate that if a neonatal patient has a significant SpO2 alarm during the last 24 hours of their stay, they are required to be monitored longer before they can be discharged."
In addition to Abington Memorial Hospital's system-wide standardization to Masimo SET pulse oximetry, the conversion also allows hospital clinicians to utilize Masimo Rainbow SET technology. Masimo Rainbow SET is an upgradable noninvasive blood constituent monitoring platform that is capable of measuring additional blood constituents that previously required invasive procedures. Masimo Rainbow SET's first application is Pulse CO-Oximetry, the first and only technology platform capable of continuously and noninvasively measuring carboxyhemoglobin (SpCO) and methemoglobin (SpMet), in addition to oxyhemoglobin (SpO2), perfusion index (PI), pleth variability index (PVI) and pulse rate.
Joe E. Kiani, CEO of Masimo, stated "Abington Memorial Hospital is a large and growing teaching hospital in the Philadelphia suburbs that is setting the pace for the next-generation of hospital physicians, specialists and clinical staff. This conversion is especially rewarding because they have chosen Masimo SET and Masimo Rainbow SET technologies to prepare their future generations of healthcare professionals. We are proud that Masimo SET is the pulse oximetry standard of care at work in Abington Memorial Hospital for today's patients and tomorrow's healthcare leaders."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Masimo SET is clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures carboxyhemoglobin (SpCO) and methemoglobin (SpMet), pleth variability index (PVI), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that Masimo SET and Masimo Rainbow SET will deliver a sufficient level of clinical improvement over alternative pulse oximetry and patient monitoring systems to allow for rapid adoption of the technology at hospitals, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended September 29, 2007, filed with the Securities and Exchange Commission on November 1, 2007. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended September 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, PVI and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


International Association of Fire Fighters (IAFF) Advocates Screening Fire Fighters for Carbon Monoxide Poisoning Using Pulse CO-Oximetry
Union believes many cardiac arrests experienced by fire fighters may be attributable to CO exposure
Irvine, California - January 8, 2008 - Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced that the International Association of Fire Fighters (IAFF) has issued education materials to more than 3,000 local union presidents in the United States and Canada calling for routine carbon monoxide (CO) screening using a Pulse CO-Oximeter for all fire fighters potentially exposed to CO. The IAFF, representing more than 287,000 full-time, professional fire fighters and emergency medical personnel who protect 85 percent of the nation's population, is the primary advocate for providing fire fighters and paramedics with the tools they need to perform their jobs, including implementation of new training programs and equipment.
In a letter to all local union presidents in North America, the IAFF highlighted the need for a new protocol whereby any fire fighter potentially exposed to CO and presenting with headache, nausea, shortness of breath, or gastrointestinal symptoms should be assessed using a Pulse CO-Oximeter. IAFF General President Harold A. Schaitberger acknowledged the prevalence, severity and frequency of the detrimental effects of CO. "We believe that many of the cardiac arrests fire fighters are experiencing may well be attributable to CO exposure," President Schaitberger said.
Because CO is present in every fire and its symptoms are nonspecific and easy to miss, the dangers of acute and prolonged CO poisoning are more pronounced for fire fighters. According to the IAFF, the risk of prolonged CO exposure during a fire does not end once the fire is controlled. The "overhaul" phase of fire control, when fire fighters seek out and extinguish any remaining fires to eliminate rekindles and stabilize both the structure and scene, can be time consuming and expose firefighters to CO levels high enough to cause death or permanent impairment. Additionally, repeated or accumulated exposures present an even greater risk to fire fighters.
Even a single high level exposure, or prolonged exposure to low levels of CO, has the potential to cause long-term cardiac, neurocognitive and psychiatric damage. The long-term effects of CO-including Parkinson-like syndromes affecting motor skills and speech, dementia, cortical blindness, acute renal failure, muscle cell death, and more-can be devastating for fire fighters and their families.
According to Mike McEvoy, EMS Director, NYS Association of Fire Chiefs; "Two facts are widely known-CO is the most common poison in the world today, and dead firefighters often have significantly elevated CO levels. The proactive use of the Pulse CO-Oximeter advocated by IAFF will help to ensure that no firefighters slip through the system with undetected CO poisoning in the line of duty."
The IAFF is the driving force behind nearly every advance in the fire and emergency services in the 20th century, from the introduction of shift schedules early in the last century to the enactment of SAFER in 2003. With recognized experts in the fields of occupational health and safety, fire-based emergency medical services and hazardous materials training, the IAFF has established professional standards for the North American Fire Service. In addition to city and county fire fighters and emergency medical personnel, the IAFF represents state employees, federal workers and fire and emergency medical workers employed at certain industrial facilities, including over 3,000 local unions in more than 3,500 communities throughout the United States and Canada.
The National Association of EMS Educators (NAEMSE) issued similar guidance to its membership recommending that EMS professionals "screen patients for carbon monoxide poisoning that have had a suspected exposure, or present with any of the signs or symptoms of carbon monoxide poisoning." These two organizations are examples of a growing trend with industry-leading emergency services associations converging toward a new standard of care for the proactive screening of CO-exposed patients and emergency services personnel by Pulse CO-Oximetry.
Joe E. Kiani, Chairman and CEO of Masimo, stated "Firefighters and EMS personnel are among our greatest heroes. They fight tirelessly to save our lives, homes, property and land from the ravages of fire-often sacrificing their own lives and health in the process. The Masimo Rad-57 Pulse CO-Oximeter was designed with saving lives and preserving health in mind. We applaud IAFF for taking this proactive step to ensure the health and well-being of the heroes who care for us all."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that the Masimo Rad-57 Pulse CO-Oximeter will deliver a sufficient level of clinical improvement over alternative devices to allow for rapid adoption of the technology, and other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended September 29, 2007, filed with the Securities and Exchange Commission on November 1, 2007. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended September 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad-57, APOD, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.
