News & Media-2007
Home / 2007
2007
NEWS & MEDIA:

Happy Holidays from Masimo!
This has been an exciting and successful year for all of us here at Masimo, and we wish to share our success with charitable organizations that you choose by making a donation to them on your behalf.
This year was particularly noteworthy for us at Masimo as it marks several key milestones, not the least of which is the shipment of our 500,000th oximeter-a Masimo Radical 7® with Masimo Rainbow SET® technology. We continued our commitment to provide clinicians with the tools they need to improve patient safety and enhance patient care with the introduction of several new products and measurements in 2007. We expanded the capabilities of our Masimo Rainbow SET technology platform with the addition of continuous and noninvasive methemoglobin (SpMet™) measurements and Pleth Variability Index (PVI®), with potential clinical applications for the noninvasive detection of hypovolemia and fluid responsiveness monitoring. In addition, we launched Masimo Patient SafetyNet™, a remote monitoring and clinician notification system to help keep patients safe on general wards that has already demonstrated its value in real-world clinical settings.

As exciting as this year has been, though, it could not have been accomplished without your efforts and support. It is your unwavering commitment to quality that energizes us, your passion for improving care that drives us, and your heroic efforts to advance patient safety that validates us.
As a show of gratitude and with a heart-felt desire to give something back to a few of the organizations that share in our global pursuit for better care and a better world, Masimo would like to donate $10 to the charity of your choice. We will make this donation in the name of each person who is an official member of Livewire as of today and who responds to this Livewire with their charity choice from the list below:
|
|
|---|
Please send us an e-mail specifying your selection to: charity@masimo.com. Only requests by e-mail to this address will be processed. We also encourage you to include any comments or suggestions you might have that will help us to better fulfill our mission and adhere to our guiding principles.
Masimo's Mission
Improve patient outcomes and reduce cost of care by taking noninvasive monitoring to new sites and applications.
Masimo's Guiding Principles
- Remain faithful to your promises and responsibilities.
- Thrive on fascination and accomplishment and not on greed and power.
- Make each day as fun as possible.
- Strive to make each year better than the year before, both personally and for the Team.
- Do what is best for patient care.
Thank You!


Masimo Chairman and CEO Joe E.
Kiani holds the 500,000th Masimo
pulse oximeter.
Masimo Celebrates 500,000th Pulse Oximeter Shipped
Alta Bates Summit Medical Center receives shipment milestone-expanding their depth and breadth of Masimo SET and Masimo Rainbow SET technologies
Irvine, California - December 21, 2007 - Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced that it has just shipped its 500,000th Masimo SET bedside pulse oximeter (excluding hand-held units) to Alta Bates Summit Medical Center, a Sutter Health network affiliate in Oakland and Berkeley, California, as part of a five-year technology expansion agreement. Following the conversion to Masimo SET pulse oximetry more than three years ago, Alta Bates Summit Medical Center recently decided to expand the relationship by adding more equipment and entering into a facility-wide site license for Masimo Rainbow SET parameters. Also this year, Sutter Health established Masimo SET as its system-wide standard for pulse oximetry.
Achieving 500,000 shipments highlights an increasing momentum in the clinical adoption of Masimo SET as the industry leading in pulse oximetry. Masimo's 200,000th shipment came in 2004, the eighth anniversary of Masimo SET shipments. The 500,000th shipment was achieved less than four years later. The growth comes as leading hospitals and health networks, like Alta Bates Summit Medical Center and Sutter Health, have recognized the superior performance of Masimo SET pulse oximetry and the clinical importance of the new, upgradeable Masimo Rainbow SET technology platform as key building blocks in helping them improve patient care and enhance patient safety initiatives.
Today, many of the world's leading hospitals have converted to Masimo SET technology, including four of the top five as listed on the US News & World Report Honor Roll, and over 50 monitoring brands have integrated Masimo SET technology into their multiparameter monitor solutions.
Nancy E. Brosnan, R.N. CCRN, Critical Care Nurse Manager, Alta Bates Summit Medical Center said, "We made the decision to move to Masimo SET pulse oximetry more than three years ago because, quite simply, the Masimo technology was a better than what was available at the time-and it has proven to be a very good product. The new capabilities of the Masimo Rainbow SET platform will provide our clinicians with a powerful new set of noninvasive measurements that will enable us to more effectively assess the cardiopulmonary status of our patients on a continuous basis, facilitating more expeditious treatment decisions that are inherently important to enhancing patient safety and improving outcomes. We couldn't afford to ignore this opportunity."
Alta Bates Summit Medical Center, a 1,094-bed not-for-profit medical center with over 218,000 outpatient visits and more than 79,000 emergency department visits annually, is a HealthGrades 2007 Distinguished Hospital Award Winner for Clinical Excellence-ranking among the top 5% in the nation for overall clinical excellence. Sutter Health is one of the nation's leading not-for-profit networks of community-based health care providers, delivering high-quality care in more than 100 Northern California communities. It is also the regional leader in infant deliveries, neonatology, orthopedics, pediatrics and cancer care services. Sutter Health supports more than 26 not-for-profit hospitals, as well as physician organizations; medical research facilities; a region-wide home health, hospice and occupational health network; and long-term care centers. A patient safety pioneer, Sutter Health network is raising the bar on patient safety with a $1.2 billion network investment in a broad range of patient safety initiatives over the next 10 years.
"Masimo SET is the right choice for our system-wide standard for pulse oximetry," said Ian Leverton, M.D., Vice President, Sutter Health Clinical Integration. "Masimo is an industry leader at the forefront of technology with superior clinical performance, and it was available at a significant financial savings."
Before the introduction of Masimo SET in 1995, pulse oximetry was reliable only when patient conditions were ideal-on motionless patients with strong pulses and good perfusion. However, in the presence of patient motion, a weak pulse or low perfusion, excessive false alarms rendered conventional pulse oximetry virtually useless.
Since then, Masimo SET Read-Through Motion and Low Perfusion pulse oximetry has become the new performance standard for leading hospitals like Alta Bates and now Sutter Health-making continuous, noninvasive monitoring by pulse oximetry more reliable and clinically-relevant than ever before. As a result, the industry has not only come to depend on continuous noninvasive physiologic monitoring by Masimo SET pulse oximetry, but industry-leading associations-like The Joint Commission (JCAHO), American Society of Anesthesiologists (ASA), and the Institute for Healthcare Improvement (IHI)-are now establishing industry standards for patient safety on general care floors based on continuous pulse oximetry monitoring in combination with appropriate clinician alerts and notification when physiologic conditions change.
Today, Masimo Rainbow SET, an upgradeable noninvasive technology platform featuring the accuracy and reliability of Masimo SET Read-Through Motion and Low Perfusion pulse oximetry, is revolutionizing patient monitoring by significantly expanding the ability to capture, track and monitor additional blood constituents that previously required invasive procedures. The first and only technology platform capable of continuously and noninvasively measuring carboxyhemoglobin (SpCO™), methemoglobin (SpMet™) and, pleth variability index (PVI), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, Masimo Rainbow SET is helping to advance patient safety and improve care.
According to Charles Van Doren, Chief of Clinical Engineering at Alta Bates Summit Medical Center, "When we first began to implement the Masimo product into our facilities over three years ago in order to standardize pulse-ox sensors, we found we were going from sensor standardization to improving our patient care. At the same time we were replacing our physiological monitors in our ICU's at the Summit campus we upgraded to Masimo. Working together with the Nurse Managers, Material Management, Clinical Engineering and Masimo Sales, we are now using only Masimo Technology throughout the medical center. The new Rainbow SET technology platform will allow us to grow as new technology becomes available."
Joe E. Kiani, Chairman and CEO of Masimo, stated "It is gratifying to see how Masimo SET and Masimo Rainbow SET technologies are transforming the clinical value and relevance of pulse oximetry by providing clinicians with a solution they can depend on and grow with. Our 500,000th shipment is a milestone that reflects the choices of the health care community-from clinicians who demand it and patient monitoring providers who work to integrate it, to hospitals and health networks, like Alta Bates Summit Medical Center and Sutter Health, that standardize on it-Masimo technologies are improving and saving lives. Alta Bates Summit Medical Center and Sutter Health are exceptional medical organizations that set their sights high on patient safety and we are proud to celebrate and share this milestone shipment with them."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Masimo SET is the most accurate and reliable pulse oximetry technology, clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures carboxyhemoglobin (SpCO) and methemoglobin (SpMet), pleth variability index (PVI), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, PVI and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at 26th Annual JPMorgan Healthcare Conference
IRVINE, Calif., Dec. 21 /PRNewswire-FirstCall/ -- Masimo Corporation (Nasdaq: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, announced today that it is scheduled to present at the 26th Annual JPMorgan Healthcare Conference at The Westin St. Francis in San Francisco, CA on Monday, January 7, 2008 at 8:30 a.m. PT. Joe E. Kiani, Chairman and CEO, and Mark P. de Raad, Executive Vice President and CFO, will be presenting.
A live Webcast of the presentation will be available on the Masimo Web site at http://www.masimo.com. A replay of the Webcast will be available following the live presentation.
About Masimo
Masimo (Nasdaq: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at http://www.masimo.com.
SOURCE Masimo Corporation
CONTACT: investors, Mark P. de Raad, Executive Vice President and Chief
Financial Officer, +1-949-297-7080, mderaad@masimo.com, or media, Tom McCall,
Vice President, Corporate Communications, +1-949-297-7075, all of Masimo
Corporation


Nationwide Respiratory Signs Preferred Pulse Oximetry Agreement with Masimo
Survey indicates most Nationwide members believed false alarms from patient motion were a significant distraction to care, emphasizing the need for more advanced pulse oximetry technology
Irvine, California - December 13, 2007 - Nationwide Respiratory and Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, announced that they have entered into a preferred provider agreement making Masimo SET pulse oximetry technology available to Nationwide's network of over 150 independent respiratory homecare providers. Masimo SET is the most accurate and reliable pulse oximetry technology, clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion.
Nationwide Respiratory, part of the VGM Group-the nation's largest member service organization for Home Medical Equipment (HME)-is a national network of over 150 independent respiratory homecare providers in the U.S. with more than 400 locations. These homecare providers use the pulse oximeter to assess the oxygen saturation of a patient during overnight sleep tests in order to determine whether or not the patient meets established Medicare or private insurance guidelines for home oxygen therapy.
Tom Pontzius, President, Nationwide Respiratory, stated "The ability to detect and capture true hypoxic events, in real-time, are essential in the administration of overnight oxygen saturation sleep tests; however, inaccurate readings and false data due to motion have hindered the effectiveness of these tests for our members. The accuracy and reliability of the pulse oximeter, even through patient motion, is crucial to effectively qualifying a patient for home oxygen therapy. Medicare guidelines cover home oxygen therapy if a patient's SpO2 drops below 89% for at least 5 minutes with a mean of 85% or less in an overnight sleep test. Getting this data correct is absolutely vital to ensuring a patient receives adequate respiratory homecare. Based on third party clinical literature and our surveys, we concluded that Masimo is by far the best choice for detecting hypoxemias and hypopneas and reducing false alarms."
Joe E. Kiani, Chairman and CEO of Masimo, said "Nationwide Respiratory is one of the nation's leading independent home respiratory provider networks. Dedicated to providing selection, service and support during the entire sleep apnea diagnosis and treatment process, Nationwide Respiratory has taken the proactive steps necessary to help its members advance the level of care they deliver. They recognized that their members have significant challenges with their current pulse oximetry solutions and were willing to invest in advanced technology, like Masimo SET, to resolve them. We are excited to partner with Nationwide Respiratory and look forward to meeting the pulse oximetry needs of their members."
As part of the contracting process, Nationwide Respiratory surveyed all its members to better understand their needs in the care of their patients with pulse oximetry. The results revealed the need for more advanced pulse oximetry technology as members overwhelmingly cited reduction in false alarms and more accurate/reliable data as reasons for their need to switch to a better pulse oximetry technology.
Reduction in False Alarms
The majority of members surveyed said that SpO2 false alarms due to patient motion are the most common type of alarms, generating the greatest number of phone calls from patients in the home. As a result, most Nationwide members surveyed said they would invest in technology if it could reduce false alarms due to motion.
Masimo SET is a breakthrough Read-Through Motion and Low Perfusion pulse oximetry technology that uses advanced breakthrough signal processing algorithms to deliver accurate and reliable SpO2 and pulse rate measurements. By delivering meaningful alarms and alerts that can be trusted to reflect a patient's true oxygenation status, clinicians can maximize their efficiency by concentrating on caring for their patients, rather than chasing false alarms.
More Accurate and Reliable Oxygen Saturation Data
Nationwide Respiratory members also confirmed that as many as 40% of overnight oximetry tests do not capture enough "real" hypoxic events and most survey respondents said they would invest in a more powerful oximetry technology if it was proven to capture more 'real' hypoxic events and improve the effectiveness of their overnight oximetry program.
Unlike conventional pulse oximeters, Masimo SET provides the greatest sensitivity (ability to detect true positives), at 98%, with the greatest specificity (the ability to reject false positives), at 97%, based on independent and objective evaluations and studies that have examined oximeter performance in real clinical environments. In fact, in a pediatric sleep laboratory setting, Masimo SET technology was found to accurately detect hypopneas 98.6% of the time, while Nellcor detected only 45.3%-missing greater than 50% of pediatric hypopneas.1
"As a result of this survey, we heard our members loud and clear and have chosen to partner with Masimo because they are the leader in oximetry technology," said Tom Pontzius, President Nationwide Respiratory.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Masimo SET is the most accurate and reliable pulse oximetry technology, clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures carboxyhemoglobin (SpCO) and methemoglobin (SpMet), pleth variability index (PVI), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
1 Differences in Pulse Oximetry Technology can Affect Detection of Sleep-Disordered Breathing in Children. Robert Brouillette, Jacinthe Lavergne, Andra Leimanis, Gillian Nixon, Sylvia Ladan, Christine McGregor. Department of Pediatrics, Montreal Children's Hospital, McGill University, Montreal, Quebec, Canada.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, PVI and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Breaking Studies at AARC Congress Show Masimo SET and Masimo Rainbow SET to be Accurate, Effective and Uniquely Beneficial in Detecting and Tracking Disease States
Orlando, Florida -- December 3, 2007 - Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, reported that multiple independent and objective clinical studies and case studies presented this week at the 2007 American Association of Respiratory Care (AARC) Annual Congress in Orlando, Florida, focused on the unique capabilities of Masimo's noninvasive patient monitoring technologies in the diagnosis, treatment and recovery of several disease states, including pulmonary effusion, methemoglobinemia, carboxyhemoglobin and hypoxemia-helping clinicians provide more rapid, improved patient care.
These new studies add to the more than 100 independent and objective studies demonstrating the superiority of Masimo SET pulse oximetry, as well as adding to the growing body of research proving the efficacy of Masimo Rainbow SET in providing accurate, reliable physiological measurements of multiple blood constituents that previously required invasive procedures. Built on Masimo SET Read-Through Motion and Low Perfusion technology, Masimo Rainbow SET is the first and only upgradeable technology platform capable of continuously and noninvasively measuring carboxyhemoglobin (SpCO), methemoglobin (SpMet) and pleth variability index (PVI), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate. Highlights of key study findings include:
New Masimo Rainbow SET SpMet Measurement Enables Rapid Diagnosis and Tracking of Benzocaine-induced Methemoglobinemia
In the case study entitled "Benzocaine Induced Methemoglobinemia After TEE," a team of Anesthesiologists headed by Dr. Mark R. Macknet at Loma Linda University in Loma Linda, California, documented the accuracy of the Masimo Rad-57 Pulse CO-Oximeter in the diagnosis and continuous monitoring of SpMet levels during a case of benzocaine-induced methemoglobinemia after transesophageal echocardiography and subsequent treatment with methylene blue. Researchers observed, 40 minutes following the benzocaine administration, a drop in the patient's oxygen saturation to 88% along with SpMet readings, as reported by the Rad-57, greater than 50%. When an arterial blood sample was analyzed by laboratory CO-Oximeter, the results reported SpMet "value over calibration limits" of the invasive laboratory CO-Oximeter. Researchers treated the patient with methylene blue using the Masimo Rad-57 Pulse CO-Oximeter "to guide the therapy" and concluded that the new Masimo Rainbow SET Pulse CO-Oximeter "has documented SpMet accuracy" enabling them to "rapidly confirm the diagnosis and continuously monitor the levels of methemoglobin."1
Masimo Rainbow SET Pulse CO-Oximetry Shown Effective in the Rapid Recognition and Continuous Measurement of Carboxyhemoglobin (SpCO)
In a report entitled "Detection of CO-Poisoning Through Pulse CO-Oximetric Measurement" by Dr. Frank Marx, Rettungsdienst, Berufsfeuerwehr Duisburg Fire Department and Emergency Service, Duisburg, Germany, the researcher used the Masimo Rainbow SET Rad-57 Pulse CO-Oximeter to quickly and accurately diagnose and triage two patients with acute CO-poisoning requiring immediate transportation to a Center of HBO (hyperbaric oxygen therapy) for treatment. Both patients had SpCO values greater than 20%, yet recovered completely following the success of immediate HBO treatment. The results of these case reports illustrate that suspected CO-poisoning "can be proven with the Masimo Rad-57 Pulse CO-Oximeter" enabling responsive "triage decisions in the field so that special treatment in an HBO facility can be arranged." Additionally, researchers concluded, "the Rad-57 Pulse CO-Oximeter provides rapid noninvasive assessment of victims and provides information that directly impacts triage and treatment decisions at the emergency scene."2
In another study entitled "Carboxyhemoglobin Levels in Smokers vs. Non-Smokers in a Smoking Environment," researchers from Ozarks Technical Community College in Springfield, Ohio, headed by Aaron Light, used the Masimo Rainbow SET Rad-57 Pulse CO-Oximeter to observe how non-smokers exposed to cigarette smoke are affected by CO-poisoning. Aided by SpCO measurements obtained from the Masimo Rad-57 Pulse CO-Oximeter, researchers tested 33 smokers (avg. SpCO of 5.04%) and 27 non-smokers (avg. SpCO of 2.49%) in an establishment where cigarette smoke was very noticeable against a control group of 50 non-smokers (avg. SpCO of 1%) in a well-ventilated non-smoking environment and found that the average SpCO level for the non-smokers in a smoking environment was nearly two and one half times higher than the control group indicating that "non-smokers are not exempt from the effects of cigarette smoke in the atmosphere."3
New Masimo Rainbow SET PVI Measurement May Provide Significant Value in the Detection and Treatment of Processes that Produce Increased Intrathoracic Pressure
In a study entitled "The Use of Pleth Variability Index (PVI) to Detect Changes in Intrathoracic Pressure," a team of Neonatologists headed by Dr. Mitchell Goldstein at the Loma Linda University Children's Hospital in Loma Linda, California, observed the correlation between Masimo PVI during pre-tap and post-tap epochs in a 6-week-old newborn with pulmonary effusion and found that PVI was significantly increased post-drainage. Study findings showed that PVI consistently increased-from 17.6 pre-tap to 21.8 post-tap in the first tap, 25.2 to 33.8 in the second, 17.6 to 20.0 in the third and 19.9 to 25.1 in the fourth-indicating that "an increase in the PVI dynamic could be correlated to the release of intrathoracic pressure." Researchers concluded, "PVI may have significant value in the diagnosis and treatment of processes that produce increased intrathoracic pressure, such as pneumothorax, chylothorax, and in this case pulmonary effusion."4
Masimo SET Read-Through Motion and Low Perfusion Pulse Oximetry More Reliable and Accurate During Air Transport
In a separate study entitled "Rad-5 and MRL SpO2 Comparison Trial: A Prospective Analysis of Pulse Oximetry During Air Transport," Jason A. Elliott of REACH, Mediplane Inc., in Santa Rosa, California, conducted a three-month prospective review of the Masimo SET Read-Through Motion and Low Perfusion Rad-5 pulse oximeter and the Welch Allyn MRL with Nellcor pulse oximetry on a total of 158 helicopter air ambulance missions and found that the Masimo Rad-5 provided more reliable and accurate SpO2 measurements during air transport and detected more real hypoxic events than the Nellcor pulse oximeter. Researchers concluded that the Nellcor pulse oximeter inside the Welch Allyn MRL had "more than two-fold increase in failure rate over the Masimo Rad-5 pulse oximeter."5
Other studies presented at AARC showed promising results for Masimo engineering prototype technologies, the Acoustic Respiration Monitoring technology (ARM) and noninvasive and continuous hemoglobin measurement (SpHb).6,7
Joe E. Kiani, Chairman and CEO of Masimo, stated, "These studies not only showcase the ability of Masimo Rainbow SET to rapidly and accurately diagnose a disease state, but to also track the progression of and recovery from that state-enabling critical real-time assessments that show whether a patient is improving or declining with intervention. This tracking and trending capability provides clinicians with the useful clinical data they need to ensure prompt administration of the most appropriate life-saving treatment. We are happy that the technologies we have developed are helping clinicians improve patient care and safety."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Masimo SET is the most accurate and reliable pulse oximetry technology, clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures carboxyhemoglobin (SpCO) and methemoglobin (SpMet), pleth variability index (PVI), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
1 Benzocaine Induced Methemoglobinemia After TEE. Mark R. Macknet, Penny L. Kimball-Jones, Richard L. Applegate, Robert D. Martin, Martin W. Allard. Anesthesiology, Loma Linda University, Loma Linda, CA.
2 Detection of CO-Poisoning Through Pulse CO-Oximetric Measurement. Frank Marx. Rettungsdienst, Berufsfeuerwehr, Duisburg, Germany.
3 Carboxyhemoglobin Levels in Smokers vs. Non-Smokers in a Smoking Environment. Aaron Light, Casie Grass, Doug Pursley, Julie Krause. Ozarks Technical Community College, Springfield, MO.
4 The Use of Pleth Variability Index (PVI) to Detect Changes in Intrathoracic Pressure. Mithcell Goldstein, Merrick Lopez, Daniel Saesim, Richard Peverini. Neonatology, Loma Linda University Children's Hospital, Loma Linda, CA.
5Rad-5 and MRL SpO2 Comparison Trial: A Perspective Analysis of Pulse Oximetry During Air Transport. Jason A. Elliot. REACH, Santa Rosa, CA.
6 Accuracy of a Novel Bioacoustic Sensor in Postoperative Patients. Mark R. Macknet, Penny L. Kimball-Jones, Richard L. Applegate, Robert D. Martin, Martin W. Allard. Anesthesiology, Loma Linda University, Loma Linda, CA.
7 Continuous Noninvasive Measurement of Hemoglobin Via Pulse CO-Oximetry During Major Surgery. Mark R. Macknet, Penny L. Kimball-Jones, Richard L. Applegate, Robert D. Martin, Martin W. Allard. Anesthesiology, Loma Linda University, Loma Linda, CA.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, PVI, SpHb, ARM, and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Corporation Given Approval to Market the Masimo Rad-57 Pulse CO-Oximeter in Japan
Masimo receives approval from Ministry of Health, Labor and Welfare (MHLW) for the revolutionary medical device that noninvasively measures carbon monoxide levels in the blood
Irvine, California - November 27, 2007 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced approval of the Masimo Rad-57 Pulse CO-Oximeter, bringing the world's first and only technology capable of noninvasively measuring carbon monoxide (CO) levels in the blood to the Japanese market-with 9,026 hospitals and Fire and Disaster Management Agency facilities1 and a population of more than 127 million.2
The Masimo Rad-57 is a fast, accurate and noninvasive way to detect elevated levels of CO in the blood, without having to draw blood and wait for costly lab results. When a clinician places the Masimo Rad-57's sensor on a patient's finger and presses a button, the device will detect the percentage of CO in the bloodstream in just seconds, allowing for prompt and possibly life-saving treatment. In addition, the Masimo Rad-57 measures SpO2 (oxygen saturation), pulse rate and perfusion index (PI) with Masimo SET technology.
Each year in Japan, it is estimated that about 2,000 people die from CO poisoning.3 An odorless and colorless toxic gas, poisoning from CO is notoriously difficult to diagnose because its symptoms mirror those of lesser afflictions, like the flu or food poisoning. Because of this, even the most skilled clinicians miss the chance to treat carbon monoxide poisoning early. As a result, unrecognized CO-poisoned patients are often unknowingly returned to the site of the exposure where they develop more serious levels of toxicity. Just one serious exposure to CO, as well as prolonged exposure to low levels of CO, can cause death or permanent brain, heart, or organ damage.4,5 Certain home CO detectors don't alarm when low levels of CO are present.
Prior to the Masimo Rad-57, the only method to accurately diagnose CO poisoning required inconvenient blood tests using expensive blood gas machines that are not available outside the hospital environment, where detection is often needed most. The ability to quickly and conveniently obtain accurate measurements in any environment leads to informed and timely treatment decisions, which can save lives and money.
"Having a noninvasive hand-held device that quickly measures the amount of CO in a person's blood would enable greater cost and care efficiencies at the scene of emergencies," said Yasuhiro Yamamoto M.D., Ph. D., Professor and Chairman, Department of Emergency and Critical Care Medicine, Nippon Medical School. "The ability to accurately diagnose and triage CO-poisoned victims at the scene can speed transport and proper treatment for higher priority cases, while reducing the flood of non-emergent patients at hospitals due to suspected poisonings and eliminating unnecessary lab tests."
Joe E. Kiani, Chairman and CEO of Masimo stated, "We are excited to bring the Masimo Rad-57 Pulse CO-Oximeter to the market in Japan where thousands of people die each year as a result of CO-poisoning and countless others suffer permanent brain, heart and organ damage. All too often CO-poisoning goes undiagnosed, silently robbing unsuspecting victims of precious health and life. The Masimo Rad-57 Pulse CO-Oximeter provides an easy and timely way to detect this deadly poison."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. Masimo SET is the most accurate and reliable pulse oximetry technology, clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced Masimo Rainbow SET, a breakthrough noninvasive blood constituent monitoring platform that can measure many blood constituents that previously required invasive procedures. Rainbow SET continuously and noninvasively measures carboxyhemoglobin (SpCO) and methemoglobin (SpMet), pleth variability index (PVI), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks relative to our estimation of the size of the Japanese market for the Rad-57 and the pace of clinical adoption of this new technology in Japan, as well as other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended September 29, 2007, filed with the Securities and Exchange Commission on November 1, 2007. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended September 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
1 Ministry of Health, Labor and Welfare (MHLW) http://www.mhlw.go.jp/toukei/saikin/hw/iryosd/05/kekka1-1.html
2 Ministry of Internal Affairs and Communications http://www.stat.go.jp/english/data/kokusei/2005/nihon/pdf/summary.pdf
3 Japan Labor Health and Welfare Organization http://www.research12.jp/d_archive/co/1_3.html
4 Weaver LK, et al. N Engl J Med, 2002;347(14):1057-067.
5 Henry CR, et al. JAMA. 2006;295(4):398-402.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, PVI and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Corporation Receives a Frost & Sullivan 2007 Industry Best Practices Award for Pulse Oximetry Leadership
Frost & Sullivan's industry research concludes, "Masimo pulse oximetry is the only vital sign module clinicians ask for by brand"
Irvine, California - November 20, 2007 - Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, announced it has received a Frost & Sullivan 2007 Industry Best Practices Award, for Pulse Oximetry Leadership - "ranking number one in the pulse oximetry industry." Frost & Sullivan further recognized "the impressive progression of Signal Extraction Technology (Masimo SET) to the 'gold standard' for reliable pulse oximetry monitoring" in honoring the company.
Superior Technology
Historically, the performance of conventional pulse oximetry, as well as its usefulness in a variety of clinical settings, has been plagued by interfering noise due to patient motion and low peripheral perfusion, which often results in false SpO2 readings and frequent alarms. "Masimo Signal Extraction Technology (SET) eliminates the issues caused by motion artifact and low peripheral perfusion through proprietary signal processing algorithms and sensor technologies," said Frost & Sullivan analyst Mike Arani in his report.
Clinical Preference
Leading clinicians around the world have put the efficacy and reliability of Masimo SET to the test in more than 100 independent and objective peer-reviewed studies that prove Masimo's revolutionary Signal Extraction Technology outperforms all others, even under the most demanding of clinical conditions. "These clinical trials have not only proved the performance superiority of Masimo SET, but time after time, they have also left a lasting impression on the participating clinicians," the report said.
Brand Recognition
Also, according to the Frost & Sullivan report, "Masimo's strong brand recognition has even driven major patient monitoring companies with in-house proprietary SpO2 technologies to offer their multiparameter monitors with the choice of Masimo SET SpO2 module." Today, Masimo SET technology is widely integrated into more than 100 multiparameter monitors and over 40 monitoring brands. "After all, performance superiority is the chief reason Masimo pulse oximetry is the only vital sign module clinicians ask for by brand when purchasing a patient-monitoring product," they added.
Market Achievements
Masimo's rapid market acceptance and market share gains were cited by Frost & Sullivan as visible examples of this combination of superior technology, clinical preference, brand recognition and exceptional brand development success.
In selecting Masimo for the 2007 pulse oximetry brand development strategy leadership award, Frost & Sullivan's analyst team tracked all the major participants in the pulse oximetry industry. The process included in-depth interviews with all market participants, customers and suppliers, along with extensive secondary and technology research to identify best practices within the industry. To determine the final ranking of competitors, Frost & Sullivan measured each on the basis of: development of unique brand strategies, competitor recognition and brand value, participation in industry trade groups, establishment of programs that allow the brand's customers to grow, and increases in customer loyalty. As a result of this ranking, Masimo topped the list of industry competitors.
According to Frost & Sullivan manager Antonio Garcia, "The key to Masimo's strong image lies in its vision of giving caregivers more options and capabilities than they expect. After all, Masimo simply takes the guess work out of pulse oximetry, so clinicians never have to guess what brand is the most reliable."
Joe E. Kiani, Chairman and CEO of Masimo, said "We are honored to receive this award from Frost & Sullivan. This recognition is especially meaningful because Frost & Sullivan has surveyed the market and responded with research findings that validate what we have heard clinicians saying all along and what our increasing marketshare momentum is a testament to-that clinicians recognize Masimo SET is the best pulse oximetry technology for their patients."
About Frost & Sullivan
Frost & Sullivan, a global growth consulting company, has been partnering with clients to support the development of innovative strategies for more than 40 years. The company's industry expertise integrates growth consulting, growth partnership services, and corporate management training to identify and develop opportunities. Frost & Sullivan serves an extensive clientele that includes Global 1000 companies, emerging companies and the investment community by providing comprehensive industry coverage that reflects a unique global perspective and combines ongoing analysis of markets, technologies, econometrics, and demographics. For more information, visit http://www.frost.com.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad-57, APOD, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at Piper Jaffray 19th Annual Healthcare Conference
Irvine, California, November 15, 2007 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, announced today that it is scheduled to present at the Piper Jaffray 19th Annual Healthcare Conference at The Pierre Hotel in New York, NY on Tuesday, November 27, 2007 at 8:30 a.m. ET. Joe Kiani, Chairman and CEO and Mark de Raad, Executive Vice President and CFO will be presenting.
A live Webcast of the presentation will be available on the Masimo Web site at www.masimo.com. A replay of the Webcast will be available approximately one hour following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Investors Contact:
Mark de Raad
Executive Vice President and
Chief Financial Officer
(949) 297-7041
mderaad@masimo.com
Media Contact:
Tom McCall
Vice President, Corporate Communications
Masimo Corporation
949-297-7075


New Research Shows Masimo Perfusion Index (PI) Effective in the Noninvasive Detection of Congenital Heart Defects (CHD) in Newborns
Findings show detection rate for CHD improved from 78% to 100% with Masimo's PI measurement
Irvine, California, November 14, 2007 - Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, reported that a new independent and objective clinical study demonstrates the ability of Masimo SET Perfusion Index (PI) to improve detection of congenital heart defects (CHD) in newborns with duct-dependent systemic circulation. Even when routine neonatal physical examinations and saturation screenings fail, PI may help to accurately detect CHD-enabling life-saving early detection and critical intervention before discharge from the hospital. It has been documented that up to 30% of all deaths from CHD in the first year of life are due to unrecognized cases being discharged to the home.
PI is a measurement featured in the Masimo Rainbow SET technology platform that reflects the real-time changes in peripheral blood flow at the monitored site and the strength of the plethysmographic signal displayed on the pulse oximeter. In addition to improving the detection of CHD in infants, the ability to noninvasively and continuously measure PI could enable faster identification of clinically significant changes in a patient's physiologic status, including potentially hypothermia, hypovolemia, shock and/or sepsis.
In the study, entitled "Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction," conducted at the Institute of Clinical Sciences, Gothenburg University, Sweden, A de-Wahl Granelli and I Ostman-Smith observed whether PI was a dependable indicator in critically-ill newborns to enable its use for congenital heart disease screening purposes. The researchers indicated that several studies have reported that babies with congenital heart disease are not detected by routine neonatal physical examinations and that neonatal screening fails mainly in children with duct-dependent systemic circulation.
Granelli and Ostman-Smith conducted single pre- and postductal measurements of PI using the Masimo Radical SET Pulse Oximeter in a total of 10,000 healthy newborns (ranging in age from 1 hour to five days) and established PI reference values of healthy babies. In establishing reference values that validate possible PI indices for normal vs. disease state in newborns, researchers were able to show that low PI values may correspond to illness.
Study results showed that combined neonatal examination and oxygen saturation screening detected only 78% of the newborns with LHOD, but when PI was added, 100% of all newborns with LHOD showed abnormalities-indicating that PI may reflect abnormal blood flow from the heart in CHD newborns. All LHOD newborns had either pre- or postductal PI values below the interquartile cut-off value of 1.18 and five had values below a potential cut-off of 0.70, leading researchers to conclude that "PI values lower than 0.70 may indicate illness and a value less than 0.50 indicates definite underperfusion." Study findings suggest that a combination of saturation screening cut-offs with PI value cut-offs may help improve the early detection of congenital heart defects that have duct-dependent systemic circulation. As a result, researchers concluded that PI is a "promising tool for improving the detection of critical congenital heart disease with duct-dependent systemic circulation."1
Joe E. Kiani, Chairman and CEO of Masimo stated, "When we introduced Masimo SET ten years ago, we knew that the improved accuracy and fidelity would allow clinicians to more tightly control the arterial oxygen levels of their patients, but it is truly remarkable to see research like this where our technology is being used to improve screening for congenital heart defects. We are all indebted to clinicians like these from Gothenburg University who are constantly looking for ways to utilize the latest technological advancements to deliver better care."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release may include forward-looking statements. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control, including: risks related to our assumption that the results of the PI study will be duplicated in future clinical studies and risks related to our assumptions regarding the correlation between PI and abnormal blood flow from the heart in CHD newborns, as well as changes in a patient's physiologic status, and other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended September 29, 2007, filed with the Securities and Exchange Commission on November 1, 2007. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended September 29, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
1 Granelli A, Ostman-Smith I. Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction. Acta Paediatrica 2007;96:1455-1459.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad-57, APOD, Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpCO, SpMet, and Pulse CO-Oximeter are trademarks or registered trademarks of Masimo Corporation.


Masimo Corporation Adopts Stockholder Rights Plan
Irvine, California, November 9, 2007-Masimo (NASDAQ: MASI) the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, announced today that the Pricing Committee of its Board of Directors has formally adopted a stockholder rights plan.
"This plan is designed to enhance the Board's ability to protect stockholders against unsolicited attempts to acquire control of the company that do not offer an adequate price to all stockholders or are otherwise not in the best interests of the company and its stockholders and customers," said Joe E. Kiani, Chairman and CEO of Masimo. "The plan is intended to provide the Board with sufficient time to consider any and all alternatives to such an action."
As disclosed in the Company's previous filings with the Securities and Exchange Commission in connection with its initial public offering, the Company anticipated adopting a stockholder rights plan following the completion of its IPO, which occurred on August 13, 2007. The Plan was not adopted in response to any attempt to acquire the Company. Under the Plan, each common stockholder of the Company at the close of business on November 26, 2007 will receive a dividend of one right for each share of the Company's common stock held of record on that date. Each right will entitle the holder to purchase from the Company, in certain circumstances described below, one one-thousandth of a share of newly-created Series A junior participating preferred stock of the Company for an initial purchase price of $136 per share. The rights' distribution will not be taxable to stockholders and the distribution of rights under the Plan will not interfere with the Company's business plans or be dilutive to or affect the Company's reported per share results.
Initially the rights will be represented by the Company's common stock certificates and will not be exercisable. The rights will generally become exercisable ten business days after any person has become the beneficial owner of 15% or more of the Company's common stock or has commenced a tender or exchange offer which, if consummated, would result in any person becoming the beneficial owner of 15% or more of the common stock of the Company.
If any person becomes the beneficial owner of 15% or more of the Company's common stock, each right will entitle the holder, other than the acquiring person, to purchase Company common stock or common stock of the acquiring person having a value of twice the exercise price. In addition, if there is a business combination between the Company and the acquiring person, or in certain other circumstances, each right that is not previously exercised will entitle the holder (other than the acquiring person) to purchase shares of common stock of the acquiring person at one-half of the market price of those shares.
The Company may redeem the rights at a price of $0.001 per right at any time prior to the date on which any person has become the beneficial owner of 15% or more of the common stock of the Company. The rights will expire on November 8, 2017, unless earlier exchanged or redeemed.
The Company will file with the Securities and Exchange Commission a Current Report on Form 8-K describing the stockholder rights plan. The Form 8-K will include a copy of the Rights Agreement governing the Plan as an exhibit.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Masimo Corporation
Investors:
Mark P. de Raad
Executive Vice President and
Chief Financial Officer
(949) 297-7080
mderaad@masimo.com
Media:
Tom McCall
Vice President, Corporate Communications
(949) 297-7075
tmccall@masimo.com
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad-57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow, SpCO, SpMet and Pulse CO-Oximeter are trademarks of Masimo Corp.


National Association of EMS Educators (NAEMSE) Recommends Screening for Carbon Monoxide Poisoning
NAEMSE Letter to Members Warns of Critical and Fatal Implications of Missing the Diagnosis of Carbon Monoxide Poisoning
Irvine, California, November 08, 2007 - Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced the National Association of EMS Educators (NAEMSE) has issued guidance to all its members advocating carbon monoxide screenings for patients presenting with any of the signs and symptoms of carbon monoxide poisoning or suspected exposure. In addition, the organization is advocating enhanced carbon monoxide training programs for all EMS professionals to help improve outcomes and save lives.
In a letter to its membership issued earlier this month, NAEMSE said failing to diagnose carbon monoxide (CO) poisoning during the emergency response efforts may lead to poor pre-hospital decisions, including failure to transport, failure to transport to an appropriate facility, failure to properly treat and failure of the emergency department to diagnose. The consequence of misdiagnosis can often result in returning the patient to a poisoned environment, possibly leading to a fatal outcome. Recognizing that CO poisoning-the most common form of poisoning in the United States-is notoriously difficult to detect, NAEMSE said improved screening and implementation of proper carbon monoxide EMS training programs "can no doubt lead to improved outcomes for patients and potentially save many lives."
Too often, even the most skilled first responders can miss the chance to treat carbon monoxide poisoning early because until now there hasn't been a fast, accurate and noninvasive way to detect elevated levels of CO in the blood. However, with the Masimo Rainbow SET Rad-57 Pulse CO-Oximeter, EMS professionals can easily detect carbon monoxide poisoning on the spot in just seconds with the push of a button, allowing for prompt and possibly life-saving treatment. In addition, Rad-57 can also limit the likelihood of long-term cardiac and neurological damage that can result from non-fatal exposures.
"We see first hand the overwhelming and immediate need for carbon monoxide screening during the first response stage and the importance of standardized carbon monoxide training protocols for EMS professionals as a matter of public safety," said NAEMSE President Angel Burba.
NAEMSE will soon have a new online training program available to all its members, free of charge, on their website www.naemse.org. The program-consisting of four carbon monoxide modules developed by Dr. Bryan Bledsoe and approved by top EMS physicians and professionals-covers the physiological dangers of CO poisoning, its signs and symptoms, as well as noninvasive methods for on-scene detection of CO in the blood. The modules include downloadable student workbooks, instructor manuals and PowerPoint slides for classroom presentation. Dr. Bryan Bledsoe is an emergency physician, highly regarded as one of the premier educators in the EMS field, and the leading author of numerous EMS textbooks.
Joe E. Kiani, Chairman and CEO of Masimo stated, "NAEMSE's recommendations for proper EMS training and field screening of carbon monoxide poisoning represents an important milestone in the establishment of new protocols for emergency responsiveness and improved public safety. If implemented nationwide, these recommendations will help reduce morbidity and mortality from unsuspected cases of carbon monoxide poisoning."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. ARM, Acoustic Respiratory Monitoring, ,Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corp.
|
Masimo Rad-57 |
 |


Community Health Network of Indianapolis Completes System-Wide Conversion to Masimo SET Pulse Oximetry Technology
Irvine, California - November 1, 2007 -- Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, announced the completion of CHN's system-wide implementation of Masimo SET pulse oximetry. Building on a four-year history of superior Masimo SET performance in other areas of its network, CHN expanded the adoption of Masimo SET pulse oximetry technology to virtually every site - making Masimo SET CHN's standard of care for precise, continuous SpO2 monitoring.
"The decision to convert our entire network to Masimo SET technology was an easy one," said Steve Erdosy, M.D., BioMed Clinical Engineer at Community Health Network. "The superior clinical performance we experienced with Masimo SET pulse oximetry in The Indiana Heart Hospital, which converted in 2003, prompted the need to have this advanced technology available in all our sites."
Ranked among the top 20 integrated health care networks in the nation, Community Health Network has more than 70 sites of care throughout central Indiana. This includes Community Hospitals East, North and South in Indianapolis and Community Hospital Anderson; The Indiana Heart Hospital, a dedicated heart hospital; Indiana Surgery Centers; Community Physicians of Indiana; Community Home Health Services; MedCheck urgent care centers; occupational health services; nursing homes; and other health care facilities. In 2006, CHN was recognized as a Performance Improvement Leader by Thomson Healthcare and, since 2001, has had fewer adverse patient safety events and discharged patients almost a day earlier.
In reaffirming the decision to convert system-wide to Masimo SET oximetry, Jan Nellinger, New Technology Analyst at Community Health Network, cited Masimo's seamless conversion as an added benefit: "The conversion was pretty invisible internally, except that the addition of the new Masimo SET pulse oximeters was a big hit with our clinical staff."
By making the conversion to Masimo, the Community Health Network joins other top hospitals in the United States-including four of the top five-as listed on the US News & World Report Honor Roll, which have all adopted Masimo SET as their primary pulse oximetry platform. Masimo SET is widely recognized as the most accurate and reliable pulse oximetry technology in the world, clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 readings even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. These studies prove Masimo SET delivers improvements in outcomes, safety and efficiency.
Community Health Network's system-wide conversion included standardizing virtually all of CHN's sites of care to Masimo SET pulse oximeters and sensors. Masimo advanced technology and sensor design increases the durability and longevity of the sensors and cables connecting the patient to the pulse oximeter. Made of a durable, non-absorbent tape material that extends the life of the sensor during single patient use, Masimo adhesive sensors have been shown to reduce sensor usage 49% to 56%1,2.
"Having an adhesive sensor with the accuracy and reliability of Masimo SET is a huge benefit that helps us to keep our patients safer with readings we can depend on, while Masimo's durability helps us to realize greater sensor efficiencies," continued Dr. Erdosy.
Joe E. Kiani, CEO of Masimo, stated "Community Health Network of Indianapolis' system-wide conversion and standardization to Masimo SET pulse oximetry is a particularly rewarding milestone that grew organically from clinical preference and adoption of Masimo SET technology within one department to virtually all departments in their network. We are proud to extend our partnership with CHN as they continue to leverage best-in-class technology to improve the quality and efficiency of healthcare in the greater Indianapolis community."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it substantially reduced false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical and laboratory studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
1 Holmes M, Thomas A, Vogt J, Gangitano E, Stephenson C, Liberman R. Useful Life of Pulse CO-Oximeter Sensors in the NICU. Respiratory Care 1998;43(10):860
2 Erler T, Avenarius S, Wichniewski E, Schmidt K, Klaber H. Longevity of Masimo and Nellcor Pulse Oximeter Sensors in the Care of Infants. J. Perinatol., 2003;23:133-135
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corporation. ARM, Acoustic Respiratory Monitoring, BiFi, Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corporation.


Masimo Reports Third Quarter 2007 Financial Results
Record results mark 17th consecutive quarter of revenue growth
Third Quarter Highlights:
* Product revenues increased 29% to a record $51.1 million
* Masimo SET pulse oximeter units increase 32% to a record 30,800 units
Irvine, California, October 30, 2007 - Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion & Low Perfusion pulse oximetry, today announced its financial results for the quarter ended September 29, 2007, with product revenues of $51.1 million representing a 29% increase over $39.8 million for the third quarter of 2006. Including royalty revenues, Masimo reported total third quarter revenues of $64.4 million compared to $57.6 million for the third quarter of 2006. Net income for the quarter was $10.6 million representing $0.16 GAAP or $0.18 non-GAAP earnings per common share.
Masimo also reported that it shipped a record 30,800 Masimo SET and Masimo Rainbow SET pulse oximeter units, excluding handheld pulse oximeters, during the third quarter of 2007, up 32% from 23,300 in the comparable prior year period, resulting in an estimated worldwide installed base of 448,000 Masimo SET pulse oximeters.
For the quarter ended September 29, 2007, due entirely to the impact of a $2.8 million, net of tax, decline in royalty payments as contemplated in our 2006 patent litigation settlement, GAAP net income declined from $12.3 million in the quarter ended September 30, 2006 to $10.6 million in the current quarter ended September 29, 2007. Masimo reported third quarter 2007 net income attributable to common stockholders of $6.9 million or $0.16 per common share as compared to $3.3 million or $0.16 per common share for the third quarter 2006. For the quarter ended September 29, 2007, on a non-GAAP basis and adjusting only for the assumed conversion of preferred stock into common stock in connection with the Masimo's recently completed initial public offering, net income was $0.18 per common share, as compared to $0.22 per common share in the prior year period.
Joe E. Kiani, Chairman and Chief Executive Officer of Masimo, said, "We are happy to report third quarter results that exceeded expectations. Our strong performance is a clear reflection of the fact that our innovative products and technologies are finding widespread adoption in virtually every segment of the clinical community. We will continue to build on these results by focusing our efforts on helping clinicians do what's best for patient care, providing them noninvasive patient monitoring technologies like our Masimo Rainbow SET platform that seek to 'solve the unsolvable' challenges in healthcare."
For the nine-month period ended September 29, 2007, Masimo's product revenues were $144.5 million, up 29% from $112.3 million in the same prior year period. Including royalty revenues, Masimo's total revenues were $187.0 million for the nine-month period ended September 29, 2007, up from $162.7 million in the same prior year period. In the nine-month period ended September 29, 2007, Masimo shipped a record 87,000 Masimo SET and Masimo Rainbow SET pulse oximeter units, excluding handheld pulse oximeters, compared to 69,900 in the same prior year period.
Net income for the nine-month period ended September 29, 2007 was $30.2 million compared to $170.9 million in the comparable prior year period, which included $262.6 million in net patent litigation settlement proceeds and various one-time stock based compensation charges related to dividend and bonus payments authorized in the first quarter of 2006. For the nine-month period ended September 29, 2007, Masimo's reported net income attributable to common stockholders was $11.8 million, or $0.40 per common share, as compared to $2.79 per common share for the nine-month period ended September 30, 2006. For the nine-month period ended September 29, 2007, on a non-GAAP basis and adjusting only for the assumed conversion of preferred stock into common stock in connection with Masimo's recently completed initial public offering, Masimo's net income was $0.54 per common share, as compared to $3.12 per share in the same prior year period.
Cash, cash equivalents and short-term investments were $88.6 million at September 29, 2007.
In August 2007, Masimo completed its initial public offering of 13,704,120 million common shares, comprised of 10,416,626 shares sold on behalf of selling stockholders and 3,287,494 shares sold on behalf of the Company, inclusive of the underwriters' full exercise of its over-allotment option. The shares were sold at $17.00 per share, for total offering of $233.0 million of which the net proceeds to the Company was approximately $47.8 million.
Financial Guidance
For the full year 2007, Masimo now expects total revenues to be approximately $250 million up from $245 million and total product revenues to be approximately $197 million up from $195 million. Masimo also expects full year GAAP earnings per share to be approximately $0.50 per share up from $0.43 per share and non-GAAP earnings per share, adjusted only for the assumed conversion of preferred stock into common stock in connection with the Company's recently completed initial public offering, to be approximately $0.63 up from $0.55. The projections and guidance set forth above are estimates only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 2:00 p.m. PT (5:00 p.m. ET) to discuss the results. The dial-in numbers are (800) 659-2037 for domestic callers and (617) 614-2713 for international callers. The reservation number for both dial-in numbers is 20647778. A live Webcast of the conference call will be available online from the "investor relations" page of the Company's corporate web site at www.masimo.com.
After the live webcast, the call will remain available on Masimo's web site through November 30, 2007. In addition, a telephonic replay of the call will be available until November 13, 2007. The replay dial-in numbers are (888) 286-8010 for domestic callers and (617) 801-6888 for international callers. Please use reservation code 39086455.
Non-GAAP Measures
Masimo prepares its consolidated financial statements in conformity with accounting principles generally accepted in the United States of America, or U.S. GAAP. In an effort to provide investors with additional information regarding the Company's results and to provide a meaningful period-over-period comparison of the Company's financial performance, the Company uses non-GAAP financial measures as defined by the Securities and Exchange Commission. The differences between the U.S. GAAP and non-GAAP financial measures are reconciled below. In presenting comparable results, the Company discloses non-GAAP financial measures when it believes such measures will be useful to investors, analysts and other interested parties in evaluating the Company's underlying business performance on a comparable basis with past and future reported earnings per share and with the financial guidance provided in this release. Management uses the non-GAAP financial measures to evaluate the Company's financial performance against internal budgets and targets. Importantly, the Company believes non-GAAP financial measures should be considered in addition to, and not in lieu of, U.S. GAAP financial measures. These non-GAAP financial measures are not based on a comprehensive set of accounting rules or principles. The Company's non-GAAP financial measures may be different from non-GAAP financial measures used by other companies.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements. All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our plans, objectives, strategies and prospects regarding, among other things, the financial condition, results of operations and business of ours and our subsidiaries; the market acceptance of products such as Patient SafetyNet; the value of new measuring new parameters such as PVI; expectations regarding our ability to design and deliver innovative new noninvasive technologies and expand into additional areas of vital signs monitoring and measurements; whether publications from prior independent clinical studies regarding PVI will be duplicated in future studies; and expectations for total revenues, product revenues, GAAP earnings per share and non-GAAP earnings per share for the full fiscal year 2007. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control including: risks related to our reliance on Masimo SET and related products for substantially all of our revenue; risks related to any failure in protecting our intellectual property; risks related to exposure to competitors' assertions of intellectual property claims; risks related to the highly competitive nature of the markets in which we sell our products; risks related to the failure to continue developing innovative products; risks related to the introduction of competing products; risks related to the lack of acceptance of new products, including the Masimo Patient SafetyNet; risks related to the loss of our customers; risks related to increases in prices for raw materials or the loss of key supplier contracts; risks related to the failure to retain senior management or replace lost senior management; risks related to product liability claims exposure; risks related to the absence of expected returns from the amount of intangible assets we have recorded; the risk that our financial performance in the fourth quarter of 2007 may not meet expectations; risks and uncertainties related to the maintenance and strength of our brand; and other factors discussed in the "Risk Factors" section of our quarterly report on Form 10-Q for the quarter ended June 30, 2007, filed with the Securities and Exchange Commission on September 20, 2007. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our quarterly report on Form 10-Q for the quarter ended June 30, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Masimo Corporation
Investors:
Mark P. de Raad
Executive Vice President and
Chief Financial Officer
(949) 297-7080
mderaad@masimo.com
Media:
Tom McCall
Vice President, Corporate Communications
(949) 297-7075
tmccall@masimo.com
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Application, Patient SafetyNet, Rainbow, SpCO, SpMet, PVI, and Pulse CO-Oximeter are registered trademarks or trademarks of Masimo Corporation.
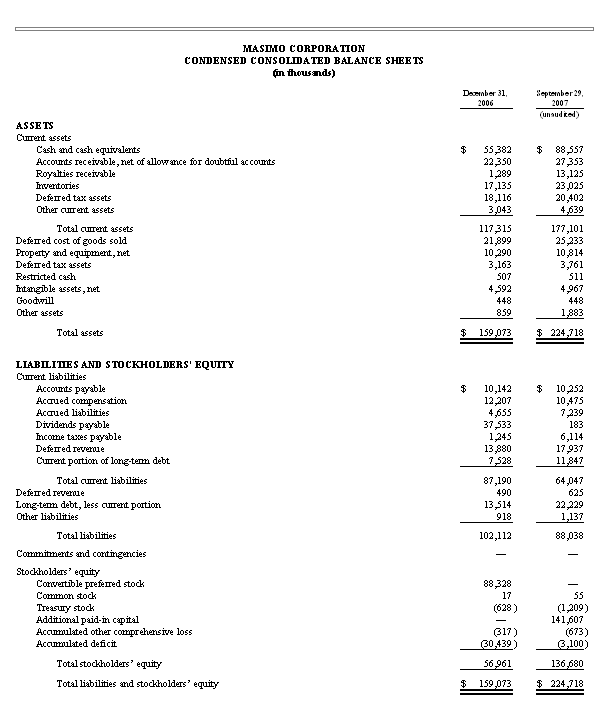
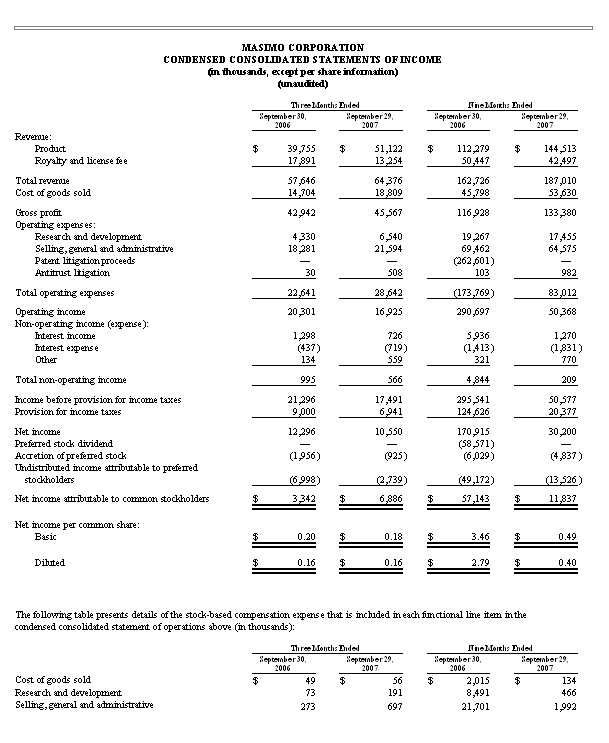
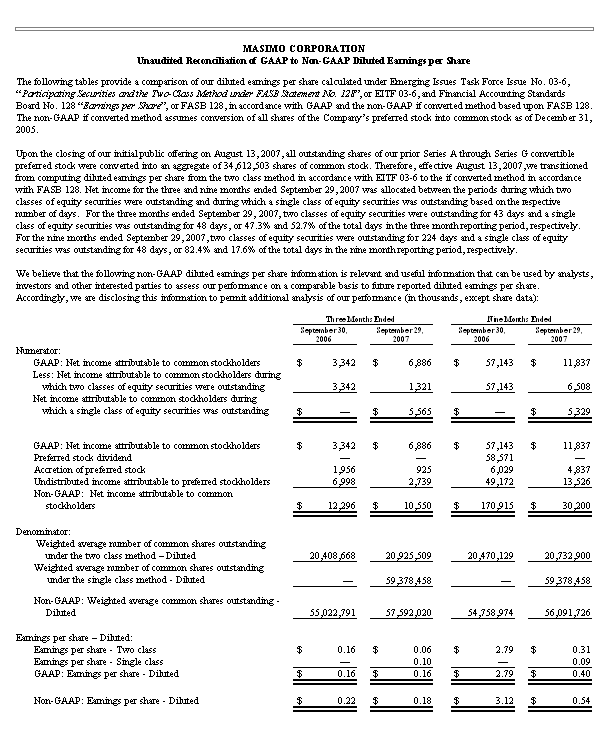


Breaking Studies--Masimo's Technologies the Focus of Multiple Clinical Studies at Last Week's American Society of Anesthesiologists' Annual Meeting
New studies show Masimo provides unique benefits in pulse oximetry, as well as noninvasive blood constituent and functional hemodynamic monitoring
Irvine, California October 25, 2007 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, reported that multiple independent and objective clinical studies and a case study were presented last week at the 2007 American Society of Anesthesiology (ASA) Annual Meeting in San Francisco focused on the unique benefits of Masimo's noninvasive patient monitoring technologies in helping clinicians provide improved patient care. These new studies add to the more than 100 independent and objective studies demonstrating the superiority of Masimo SET pulse oximetry, as well as adding to the growing body of research proving the efficacy of Masimo Rainbow SET in providing accurate, reliable physiological measurements of multiple blood constituents that previously required invasive procedures.
Built on Masimo SET Read-Through Motion and Low Perfusion technology, Masimo Rainbow SET is the first and only upgradeable technology platform capable of continuously and noninvasively measuring carboxyhemoglobin (SpCO), methemoglobin (SpMet) and pleth variability index (PVI), in addition to oxyhemoglobin (SpO2), perfusion index (PI) and pulse rate. Highlights of key study findings include:
Masimo SET and Blue Sensor more accurate and more reliable in cyanotic children
In a study entitled "New Generation and Old Generation Pulse Oximeters in Children with Cyanotic Congenital Heart Disease" a team of anesthesiologists headed by Dr. Maxime Cannesson at the Louis Pradel Hospital in Lyon, France, found that in a study of 10 children ages seven days to 53 months following palliative cardiac surgery, Masimo obtained readings 100% of the time and "Nellcor failed to obtain readings 15% of the time." Additionally, findings showed a "significant difference between the Nellcor SpO2 reading and the blood gas readings," but no significant difference between the Masimo and blood gas readings when compared to laboratory CO-Oximetry. For the 116 arterial samples, the accuracy of the Masimo Radical pulse oximeter was two times better than that of the Nellcor N-395 pulse oximeter, leading researchers to conclude that "new generation pulse oximeters provide more accurate information and are more reliable than old generation pulse oximeters."1
A separate study, entitled "New Pulse Oximetry Sensors with Low Saturation Accuracy Claims - A Clinical Evaluation" performed by Dr. Peter Cox at the Hospital for Sick Children in Toronto, Canada, compared the accuracy of the Masimo Radical with Blue Sensor, the Nellcor N-600 (with Lo-Sat) and Max-I sensor, and the Radical with LNOP sensor to laboratory CO-Oximetry readings on 12 patients with congenital cyanotic cardiac lesions (CCCL) and concluded that "despite advances in technology, only the Masimo Blue sensor demonstrates acceptable accuracy."2 Findings showed that, although the Nellcor N-600 with LoSat is advertised to work in CCCL patients, the accuracy demonstrated in this study was not within Nellcor's published accuracy specifications; whereas the Masimo Radical with Blue Sensor had significantly better accuracy and was within Masimo specifications.
Masimo Rainbow SET provides a noninvasive tool to greatly facilitate the clinical diagnosis and treatment for carbon monoxide poisoning and Benzocaine-induced methemoglobinemia
In a report entitled "Rad-57 Rainbow CO-Oximeter in Detecting Methemoglobin during Upper GI Endoscopy - A Case Report" by Dr. Udaya B. Padakandla at Baylor University Medical Center in Dallas, Texas, the researcher described how four minutes after the Benzocaine application, the patient exhibited a rise in methemoglobin levels above 40% along with a decrease in SpO2 (to 90%), both measured continuously with the Masimo Rainbow SET Rad-57 and confirmed via arterial blood gas analysis. The patient was treated for methemoglobinemia with methylene blue and the SpO2 and methemoglobin levels returned to normal. This case report documents the induction of methemoglobinemia in a GI patient from Benzocaine spray used as a topical anesthetic prior to endoscopy and shows the accuracy and effectiveness of Masimo Rainbow SET to continually and noninvasively measure methemoglobin levels during the entire procedure as confirmed by blood gas with CO-oximetry. This case illustrates the importance of continuous monitoring of SpMet when Benzocaine spray is used.3
In another study entitled "Clinical Analyses of 429 Cases of Acute CO Poisoning," researchers from the Hyperbaric Oxygen Department of Beijing Chaoyang Hospital in Beijing, China, headed by Dr. Zhou Li, used the Masimo Rainbow SET Rad-57 Pulse CO-Oximeter and found that 98 of the 429 acute carbon monoxide (CO) poisoning patients had methemoglobin (MetHb) greater than 1.2 percent at the first measurement with significant positive correlation between CO and MetHb levels, suggesting that "MetHb may be involved in the physiopathological process of hypoxia of acute CO poisoning patients." The application of Masimo Rainbow SET technology in the noninvasive measurement of carbon monoxide and methemoglobin concentration levels in the blood lead researchers to conclude that this technology "provides a noninvasive tool to greatly facilitate the clinical diagnosis and treatment for CO poisoning patients."4
In a study entitled "A Comparison of Finger, Ear and Forehead SpO2 on Detecting Oxygen Desaturation in Healthy Volunteers," by Dr. Kentaro Tokuda and researchers from the Kyushu University Hospital in Fukuoka City, Japan, researchers compared the time for desaturations and resaturations to be detected by Nellcor forehead (MaxFast), Masimo ear (TC-I) and finger (LNOP) SpO2 sensors when patients were in heads-down, supine and heads-up positions. The study found Masimo's ear sensor to be as fast, or slightly faster, than the Nellcor forehead sensor in detecting desaturations and resaturations. The researchers concluded "sensors on the head (forehead and ear), especially Masimo ear sensor, can detect hypoxia as soon as or even sooner than finger," adding that "sensors on the head (forehead and ear) can also sense recovery state from hypoxia sooner than finger."5
In addition, a study entitled "New Algorithm for Automatic Estimation of the Respiratory Variations in the Pulse Oximeter Waveform" conducted at the Louis Pradel Hospital in Lyon, France by a team headed by Dr. Maxime Cannesson demonstrated the ability of Pleth Variability Index (PVI) to accurately and noninvasively detect changes in patient fluid volume. Researchers noted that PVI "has potential clinical applications for noninvasive hypovolemia detection and fluid responsiveness monitoring."6
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
1 New Generation and Old Generation Pulse Oxymeters in Children with Cyanotic Congenital Heart Disease. Maxime Cannesson, M.D., Jean Neidecker, M.D., Dominique Bompard, M.D., Catherine Vedrinne, M.D., Ph.D., Jean-Jacques Lehot, M.D. Anesthesiology, Hospices Civils de Lyon, Louis Pradel Hospital, Lyon, France.
2 New Pulse Oximetry Sensors with Low Saturation Accuracy Claims - A Clinical Evaluation. Peter N. Cox, M.D., F.R.C.P.C. Department of Critical Care Medicine, Hospital for Sick Children, Toronto, Ontario, Canada.
3 Rad-57 Rainbow CO-Oximeter in Detecting Methemoglobin during Upper GI Endoscopy - A Case Report. Udaya B. Padakandla, M.B.B.S., F.F.A.R.C.S.I. Anesthesiology, Baylor University Medical Center, Dallas, Texas.
4 Clinical Analyses of 429 Cases of Acute CO Poisoning. Zhou Li, M.M.S., Chunjin Gao, B.S.M.E., Xiang Huang, M.M.S., Gang Wang, M.D., Huan Ge, B.S.M.E. Hyperbaric Oxygen Department, Beijing Chaoyang Hospital Affiliate of Capital University of Medical Sciences, Beijing, China.
5 A Comparison of Finger, Ear and Forehead SpO2 on Detecting Oxygen Desaturation in Healthy Volunteers. Kentaro Tokuda, M.D., Kengo Hayamizu, M.D., Kei Ogawa, M.D., Takeshi Hirai, M.D., Kazuo Irita, M.D., Ph.D. Anesthesiology and Critical Care Medicine, Kyushu University Hospital, Fukuoka City, Japan.
6 New Algorithm for Automatic Estimation of the Respiratory Variations in the Pulse Oximeter Waveform. Maxime Cannesson, M.D., Bertrand Delannoy, M.D., Antoine Morand, M.D., Olivier Bastien, M.D., Ph.D., Jean-Jacques Lehot, M.D., Ph.D. Anesthesiology, Hospices Civils de Lyon, from Louis Pradel Hospital, Lyon, France.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. ARM, Acoustic Respiratory Monitoring, BiFi, Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corp.


Masimo to Report Third Quarter 2007 Financial Results on October 30, 2007
Conference call and webcast with Masimo CEO and CFO to begin after markets close at 2:00 pm PT (5:00 pm ET)
IRVINE, Calif., October 18, 2007-Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced it will release third quarter financial results for the period ended September 29, 2007 after the market closes on Tuesday, October 30, 2007.
A conference call to review the results will begin at 2:00 p.m. PT (5:00 p.m. ET) and will be hosted by Joe E. Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President & Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the Company's corporate web site at www.masimo.com. The dial-in numbers are (800) 659-2037 for domestic callers and (617) 614-2713 for international callers. The reservation number for both dial-in numbers is 20647778. After the live webcast, the call will remain available on Masimo's web site through November 30, 2007. In addition, a telephonic replay of the call will be available until November 13, 2007. The replay dial-in numbers are (888) 286-8010 for domestic callers and (617) 801-6888 for international callers. Please use reservation code 39086455.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Mark P. de Raad
Executive Vice President and
Chief Financial Officer
(949) 297-7080
mderaad@masimo.com
Media Contact:
Tom McCall
Vice President, Corporate Communications
Masimo Corporation
949-297-7075
tmccall@masimo.com
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. ARM, Acoustic Respiratory Monitoring, BiFi, Rainbow, Patient SafetyNet, SpCO, SpMet, SpHb, PVI and Pulse CO-Oximeter are trademarks of Masimo Corp.


Breaking Study: Masimo Pleth Variability Index (PVI) Shown Effective in Noninvasive Detection of Changes in Ventricular Preload and Fluid Volume
Most recent addition to Masimo Rainbow SET platform seen as having potential clinical application for the noninvasive detection of hypovolemia and fluid responsiveness monitoring
San Francisco, California October 15, 2007 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, reported that a new independent and objective clinical study presented today at the 2007 American Society of Anesthesiologists (ASA) Meeting in San Francisco demonstrated the ability of Pleth Variability Index (PVI) to accurately and noninvasively detect changes in ventricular preload. The newest addition to the Masimo Rainbow SET technology platform, PVI is a continuous and noninvasive quantified measurement of changes in the perfusion index, capturing volume changes that may compromise cardiac function and affect systemic circulation.
The study entitled "New Algorithm for Automatic Estimation of the Respiratory Variations in the Pulse Oximeter Waveform", was conducted at the Louis Pradel Hospital in Lyon, France, by a research team headed by Dr. Maxime Cannesson. The research team indicated that while respiratory variations in the pulse oximeter plethysmography waveform amplitude are sensitive to changes in preload and can predict fluid responsiveness in mechanically ventilated patients, they previously were not easily measured noninvasively from a bedside monitor. However, a new algorithm, PVI, available in the Masimo Rainbow SET technology platform may provide a new method for noninvasively predicting fluid responsiveness.
In this study, Dr. Cannesson and the research team tested the ability of PVI to detect changes in ventricular preload in 20 vascular surgery patients under mechanical ventilation. Mean arterial pressure (MAP) and central venous pressure (CVP) via arterial catheter and central venous catheter were recorded along with PVI at baseline and while patients were in head-down and head-up positions. Researchers stated, this study is the "first to demonstrate the ability of PVI, an index automatically derived from the pulse oximeter waveform analysis, to detect changes in ventricular preload. This new index has potential clinical applications for noninvasive hypovolemia detection and fluid responsiveness monitoring."1
"Fluid management optimization in mechanically ventilated patients undergoing anesthesia is of major importance since it may have clinical and economical impact," Dr. Cannesson stated. "Indicators of response to volume expansion relying on cardio-pulmonary interactions are the best predictors of fluid responsiveness. Ideally, continuous noninvasive monitoring of these parameters would provide valuable information to clinicians in caring for mechanically ventilated patients. Masimo's new derived index, PVI, appears to offer such a continuous, noninvasive monitor."
Masimo PVI is a unique new measurement featured in the Masimo Rainbow SET technology platform that quantifies changes in perfusion index. PVI displays a numeric representation of the changes as a percentage on the Rainbow SET oximeter, thus allowing clinicians to track and trend these changes over time. A rising PVI may indicate developing hypovolemia (an abnormally low volume of blood circulating through the body) and a falling PVI post-fluid resuscitation is evidence of an appropriate fluid responsiveness. This trending may aid in determining appropriate intervention for patients experiencing physiologic changes in fluid volume and cardiac function, leading to better patient outcomes.
Appropriate fluid levels are vital to reducing postoperative risks and improving patient outcomes as fluid volumes that are too low (under hydration) or too high (over hydration) have been shown to decrease wound healing, increase risk of infection and cardiac complications. In extreme cases, severe hypovolemia can lead to hypovolemic shock as peripheral circulation shuts down to preserve central circulation in an attempt to maintain the heart, brain and kidney functions.
In commenting on the study, Dr. Kevin K. Tremper, Chairman of Anesthesia at University of Michigan said, "One of the biggest challenges we have in the operating room is determining whether, from a fluid volume perspective, a patient is full or empty. If PVI can help us noninvasively determine this in real time, PVI will be a huge benefit to clinicians and positively impact patient care and safety."
Built on Masimo SET Read-Through Motion and Low Perfusion technology, Masimo Rainbow SET is the first and only upgradeable technology platform capable of continuously and noninvasively measuring carboxyhemoglobin (SpCO) and methemoglobin (SpMet), in addition to oxyhemoglobin (SpO2), perfusion index (PI), Pleth Variability Index (PVI) and pulse rate.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
1 New Algorithm for Automatic Estimation of the Respiratory Variations in the Pulse Oximeter Waveform. Maxime Cannesson, M.D., Bertrand Delannoy, M.D., Antoine Morand, M.D., Olivier Bastien, M.D., Ph.D., Jean-Jacques Lehot, M.D., Ph.D. Anesthesiology, Hospices Civils de Lyon, from Louis Pradel Hospital, Lyon, France.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. ARM, Acoustic Respiratory Monitoring, BiFi, Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corp.
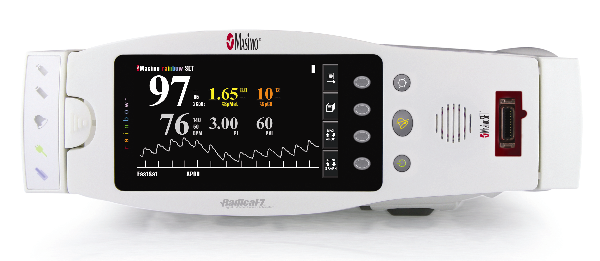


Masimo Launches Patient SafetyNet and Showcases the Rainbow SET Upgradeable Noninvasive Monitoring Platform at 2007 ASA Annual Meeting
The New Masimo Patient SafetyNet and the Innovative, Upgradeable Masimo Rainbow SET Technology Platform Will Help Clinicians Meet Standards Bodies' Calls to Improve Patient Safety
Irvine, California -- October 3, 2007 - Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced it will launch Masimo Patient SafetyNet, a new remote monitoring and clinician notification system, and showcase its upgradeable Masimo Rainbow SET technology platform at the 2007 American Society of Anesthesiologists (ASA) Annual Meeting in San Francisco, California on October 13, 2007.
Masimo Patient SafetyNet is an easy-to-use system that combines the performance of Masimo SET pulse oximetry with wireless clinician notification via pager to provide a new level of safety to patients on general care floors, where nurse-to-patient ratios preclude the level of direct surveillance required and recommended to preempt sentinel events. Numerous standards bodies, including the Joint Commission (JCAHO), the American Society of Anesthesiologists, the Anesthesia Patient Safety Foundation and the Institute for Healthcare Improvement are calling for improved patient safety standards on general care floors.
Masimo Patient SafetyNet is specifically designed to keep at-risk patients safe on general care floors by connecting them to qualified caregivers quickly, easily, and accurately. When a Patient SafetyNet-monitored patient is in respiratory distress, meaningful and actionable alarms are generated by the Masimo bedside monitor and sent wirelessly to designated clinicians for review and response. As a result, appropriate clinical intervention can be initiated quickly, preempting sentinel events-defined by the Joint Commission as "an unexpected occurrence involving death or serious physical injury not related to the natural course of the patient's illness."
The Masimo Patient SafetyNet system instantly routes bedside-generated alarms through a server to a qualified clinician's hand-held paging device in real-time. For added safety, the system allows for automatic escalation of the alarm to additional clinicians when necessary. Each Masimo Patient SafetyNet system can support up to 40 bedside monitors and can either be integrated into a hospital's existing IT infrastructure or operate as a stand-alone wireless network.
The Accuracy of Masimo SET Pulse Oximetry Enables Monitoring on General Care Floors
At the heart of Masimo Patient SafetyNet is Masimo SET pulse oximetry, clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 and pulse rate measurements, even under the most difficult clinical conditions of patient motion and low peripheral perfusion. Previous attempts at developing general care floor monitoring systems were unsuccessful because the high volume of false pulse oximetry alarms generated by conventional pulse oximetry led systems to provide false alarms so often that clinicians ultimately ignored all alarms. Masimo SET reduced false alarms by over 90% and improved detection of true alarms to over 97%, delivering accurate and reliable alarms and alerts that can be trusted to reflect a patient's true oxygenation status. This accuracy and reliability enables clinicians on general care floors to maximize their efficiency and increase patient safety by responding with confidence to alarms knowing they represent real clinical events, without being burdened by false alarms.
"The clinical realities of today's general care floors have changed-most notably due to the increased risk of sentinel events resulting from aggressive post-operative pain management, an increasing number of patients at risk for Obstructive Sleep Apnea (OSA) and increased co-morbidities at admission. These and other issues fundamentally challenge the way in which care is delivered," explained Masimo Chairman and CEO Joe E. Kiani. "The combination of these patient care challenges with the decrease in nurse-to-patient ratios has resulted in increased populations of higher acuity patients on general care floors-prompting industry standards bodies to drive a new standard of care that calls for continuous monitoring of patient oxygen levels with pulse oximetry, with clinically meaningful alarms sent to appropriate caregivers. Masimo Patient SafetyNet helps clinicians meet these emerging care standards."
Masimo Rainbow SET Platform Enables Clinicians to Upgrade to New Features and Measurements
In addition to Masimo Patient SafetyNet, the company will be showcasing the Masimo Rainbow SET Platform, an innovative, upgradeable multi-functional technology platform enabling noninvasive, continuous blood constituent and functional hemodynamic monitoring. The Masimo Rainbow SET Platform allows clinicians to choose the features and measurements needed to meet their specific clinical requirements, and then upgrade to additional functionality whenever they choose. Masimo Rainbow devices come standard with Masimo SET pulse oximetry, providing accurate and reliable SpO2, pulse rate and perfusion index measurements, even in the most difficult clinical conditions. In addition, clinicians can choose to upgrade to additional measurements including continuous noninvasive carbon monoxide (SpCO) and methemoglobin (SpMet) monitoring and Pleth Variability Index (PVI) simply by purchasing field-installed software upgrades whenever they need them.
When using Masimo Rainbow SET technology, clinicians can more accurately determine their patients' true oxygenation status by measuring levels of SpMet and SpCO in the blood, two potentially fatal dyshemoglobins proven to increase morbidity and mortality in a broad range of clinical settings.
By continuously, noninvasively and accurately determining levels of SpMet, clinicians can accurately determine if drugs they are administering are causing methemoglobinemia. The drugs that have been shown to contribute to methemoglobinemia include Benzocaine, Cetacaine and Prilocaine, Celecoxib, Dapsone, EMLA Creams, Flutamide, Nitrates, Nitric Oxide, Nitroglycerin, Sodium Nitroprusside, Sodium Nitrate and Sulfonamides.
In a surgical setting, continuous monitoring of SpCO can allow clinicians to detect elevated levels of carboxyhemoglobin that can be caused by everything from desiccated soda lime ("Monday Morning Phenomena") or other poisons during surgery to smokers reporting for surgery with high SpCO values. Increased SpCO compromises healing and may lead to death, and smokers with residual elevated COHb at the time of anesthesia are at cardiac ischemic risk.
Also for the first time at the ASA, Masimo will be showing its latest Rainbow measurement, Pleth Variability Index, or PVI. The ability to noninvasively measure and trend PVI, a continuous noninvasive quantified measurement of changes in the perfusion index that could be indicative of blood volume status, provides additional indicators of a patient's physiologic condition, including the patient's level of hydration. This allows for more precise, timely diagnosis and better treatment decisions that lead to improved patient outcomes.
"At Masimo, we are wholly dedicated and committed to developing products and technologies that enable clinicians to deliver the best possible care and to increase the safety of their patients," Mr. Kiani added. "Since our beginnings nearly 20 years ago, we have been focused on improving patient outcomes and reducing the cost of care by bringing noninvasive monitoring to new sites and new applications. Masimo Patient SafetyNet and the Masimo Rainbow SET platform are clear examples of this ongoing commitment."
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. ARM, Acoustic Respiratory Monitoring, BiFi, Rainbow, Patient SafetyNet, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corp.
INTRODUCING MASIMO PATIENT SAFETYNET
Remote Patient Monitoring and Clinician Notification System

The easy-to-use monitoring system designed specifically to reduce sentinel events on general care floors by wirelessly linking at-risk patients to appropriately designated clinicians


Masimo Reports Second Quarter 2007 Financial Results
Record results mark 16th consecutive quarter of revenue growth
Second Quarter Highlights:
- Product revenues increase 26% to a record $47.6 million
- A record 29,600 Masimo SET pulse oximeter units shipped
- Net income of $10.6 million--$0.13 GAAP EPS / $0.19 Non-GAAP EPS
Irvine, California, September 19, 2007 - Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced its financial results for the quarter ended June 30, 2007, with product revenues of $47.6 million representing a 26% increase over $37.8 million for the second quarter of 2006. Including royalty revenues, Masimo reported total second quarter revenues of $63.7 million compared to $55.8 million for the second quarter of 2006. Net income for the quarter was $10.6 million, representing $0.13 GAAP or $0.19 non-GAAP earnings per common share.
Masimo also shipped a record 29,600 Masimo SET and Masimo Rainbow SET pulse oximeter units, excluding handheld pulse oximeters, during the second quarter of 2007, up from 23,700 in the comparable prior year period, resulting in an estimated worldwide installed base of 424,000 Masimo SET pulse oximeters.
For the quarter ended June 30, 2007, Masimo's net income was $10.6 million compared to $13.9 million for the same quarter in 2006. These results reflect expected decreases in royalty revenue and interest income resulting from the January 2006 patent litigation settlement and related dividend distributions, as well as planned increases in research and development, sales, marketing and general and administrative expenses to support additional product development efforts and the Company's global business expansion. Under the two class method for computing and reporting earnings per share, the Company reported second quarter 2007 net income attributable to common stockholders of $2.8 million or $0.13 per common share based on weighted average common shares outstanding of 20,732,014 as compared to $3.9 million or $0.19 per common share based on weighted average common shares outstanding of 20,301,629 for the second quarter 2006. For the quarter ended June 30, 2007, on a non-GAAP basis and adjusting only for the assumed conversion of preferred stock into common stock in connection with the Company's recently completed initial public offering, net income was $0.19 per common share based on weighted average common shares outstanding of 55,344,517 as compared to $0.25 per common share based on weighted average common shares outstanding of 54,914,132 in the prior year period.
Joe E. Kiani, Chairman and Chief Executive Officer of Masimo, said, "We are happy to report a record second quarter with solid product revenue growth and net income that exceeded expectations. These results reflect the clinical community's recognition of the benefits of our breakthrough read-through motion pulse oximetry, our strong customer service, and continued commitment to innovation, as evidenced by our new Rainbow SET platform."
For the six-month period ended June 30, 2007, Masimo's product revenues were $93.4 million, up 29% from $72.5 million in the same prior year period. Including royalty revenues, Masimo's total revenues were $122.6 million for the six-month period ended June 30, 2007, up from $105.1 million in the prior year period. In the six-month period ended June 30, 2007, Masimo shipped a record 56,200 Masimo SET and Masimo Rainbow SET pulse oximeter units, excluding handheld pulse oximeters, compared to 46,600 in the same prior year period.
Net income for the six-month period ended June 30, 2007 was $19.7 million compared to $158.6 million in the comparable prior year period, which included $262.6 million in net patent litigation settlement proceeds and various one-time stock-based compensation charges related to dividend and bonus payments authorized in the first quarter of 2006. Under the two class method for computing and reporting earnings per share for the six-month period ended June 30, 2007, Masimo reported net income attributable to common stockholders was $5.1 million, or $0.25 per common share based on weighted average common shares outstanding of 20,669,111, as compared to $2.67 per common share based on weighted average common shares outstanding of 19,975,936 for the six-month period ended June 30, 2006. For the six-month period ended June 30, 2007, on a non-GAAP basis and adjusting only for the assumed conversion of preferred stock into common stock in connection with Masimo's recently completed initial public offering, Masimo's net income was $0.36 per common share based on total weighted average common shares of 55,311,614 as compared to $2.91 per share based on weighted average common shares outstanding of 54,588,439 in the prior year period.
Cash, cash equivalents and short-term investments were $35.0 million at June 30, 2007.
In August 2007, Masimo completed its initial public offering of 13,704,120 million common shares, comprised of 10,416,626 shares sold on behalf of selling stockholders and 3,287,494 shares sold on behalf of the Company, inclusive of the underwriters' full exercise of its over-allotment option. The shares were sold at $17.00 per share, for net proceeds to the Company of approximately $47.0 million.
During the first six-months of 2007, Masimo introduced a variety of new products including the new color Radical-7 and its latest measurement, Pleth Variability Index (PVI). The Radical-7 comes standard with the Rainbow SET MX circuit board which allows for upgrades to additional measurements through purchased software upgrades. PVI is a noninvasive measurement of functional hemodynamics, which clinicians have indicated may be valuable in monitoring fluid responsiveness and cardiac function. In the second quarter, Masimo also introduced its line of Rainbow single patient use adhesive sensors for adults, children, infants and neonates. The Rainbow sensors are capable of measuring not only oxygen saturation (SpO2), Pulse Rate and Perfusion Index, but also, carboxyhemoglobin (SpCO), methemoglobin (SpMet) and PVI. In June 2007, Masimo received U.S. Food & Drug Administration 510(k) clearance expanding its claims for SpMet monitoring to cover neonatal patients with its neonatal and infant Rainbow adhesive sensors. The ability to continuously and noninvasively measure methemoglobin levels in neonatal patients is especially important due to the use of inhaled nitric oxide (iNO) therapy to treat hypoxic respiratory failure in newborns, which has been shown to induce methemoglobinemia.
Financial Guidance
For the full year 2007, Masimo expects total revenues to be approximately $245 million and total product revenues to be approximately $195 million. The Company expects full year GAAP earnings per share to be approximately $0.43 and non-GAAP earnings per share, adjusted only for the assumed conversion of preferred stock into common stock in connection with Masimo's recently completed initial public offering, to be approximately $0.55. The projections and guidance set forth above are estimates only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 2:00 p.m. PT (5:00 p.m. ET) to discuss the results. The dial-in numbers are (866) 700-7441 for domestic callers and (617) 213-8839 for international callers. The reservation number for both dial-in numbers is 11107851. A live Webcast of the conference call will be available online from the "investor relations" page of the Company's corporate web site at www.masimo.com.
After the live webcast, the call will remain available on Masimo's web site through October 19, 2007. In addition, a telephonic replay of the call will be available until October 3, 2007. The replay dial-in numbers are (888) 286-8010 for domestic callers and (617) 801-6888 for international callers. Please use reservation code 65525346.
Non-GAAP Measures
Masimo prepares its consolidated financial statements in conformity with accounting principles generally accepted in the United States of America, or U.S. GAAP. In an effort to provide investors with additional information regarding the Company's results and to provide a meaningful period-over-period comparison of the Company's financial performance, the Company uses non-GAAP financial measures as defined by the Securities and Exchange Commission. The differences between the U.S. GAAP and non-GAAP financial measures are reconciled below. In presenting comparable results, the Company discloses non-GAAP financial measures when it believes such measures will be useful to investors, analysts and other interested parties in evaluating the Company's underlying business performance on a comparable basis with past and future reported earnings per share and with the financial guidance provided within this press release. Management uses the non-GAAP financial measures to evaluate the Company's financial performance against internal budgets and targets. Importantly, the Company believes non-GAAP financial measures should be considered in addition to, and not in lieu of, U.S. GAAP financial measures. These non-GAAP financial measures are not based on a comprehensive set of accounting rules or principles. The Company's non-GAAP financial measures may be different from non-GAAP financial measures used by other companies.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements. All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our plans, objectives, strategies and prospects regarding, among other things, the financial condition, results of operations and business of ours and our subsidiaries; our expectations regarding the value of monitoring methemoglobin levels in neonatal patients; the value of new measurements such as PVI; expectations regarding our ability to design and deliver innovative new noninvasive technologies and expand into additional areas of vital signs monitoring and measurements, including carbon monoxide, methemoglobin and PVI; and expectations for total revenues, product revenues, GAAP earnings per share and non-GAAP earnings per share for the full fiscal year 2007. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control including: risks related to our reliance on Masimo SET and related products for substantially all of our revenue; risks related to any failure in protecting our intellectual property; risks related to exposure to competitors' assertions of intellectual property claims; risks related to the highly competitive nature of the markets in which we sell our products; risks related to the failure to continue developing innovative products; risks related to the introduction of competing products; risks related to the lack of acceptance of new products; risks related to the loss of our customers; risks related to increases in prices for raw materials or the loss of key supplier contracts; risks related to the failure to retain senior management or replace lost senior management; risks related to product liability claims exposure; risks related to the absence of expected returns from the amount of intangible assets we have recorded; the risk that our financial performance in the third and fourth quarters of 2007 may not meet expectations; risks and uncertainties related to the maintenance and strength of our brand; and other factors discussed in the "Risk Factors" section of our Prospectus dated August 7, 2007 filed with the Securities and Exchange Commission. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our Prospectus dated August 7, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Masimo Corporation
Investors:
Mark P. de Raad
Executive Vice President and Chief Financial Officer
(949) 297-7080
mderaad@masimo.com
Media:
Tom McCall
Vice President, Corporate Communications
(949) 297-7075
tmccall@masimo.com
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Application, ARM, Acoustic Respiratory Monitoring, Rainbow, SpCO, SpMet, PVI, SpHb and Pulse CO-Oximeter are registered trademarks or trademarks of Masimo Corporation.
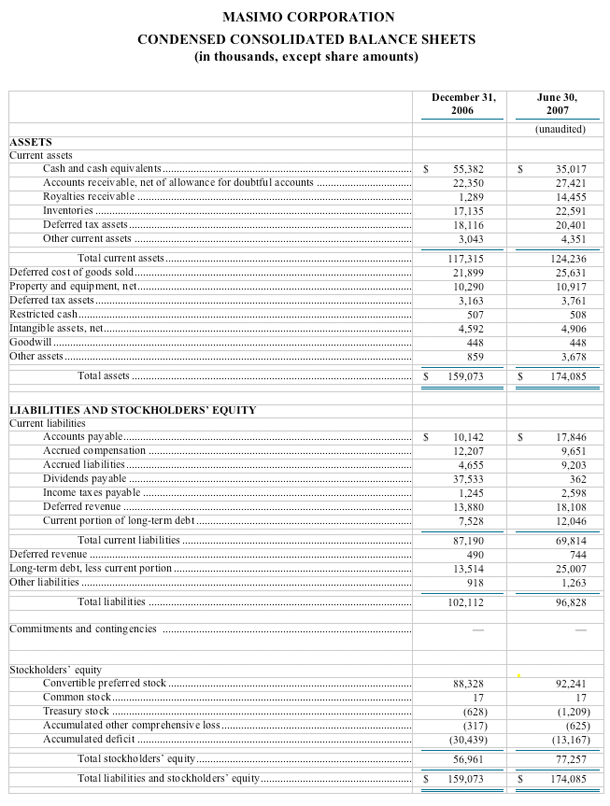
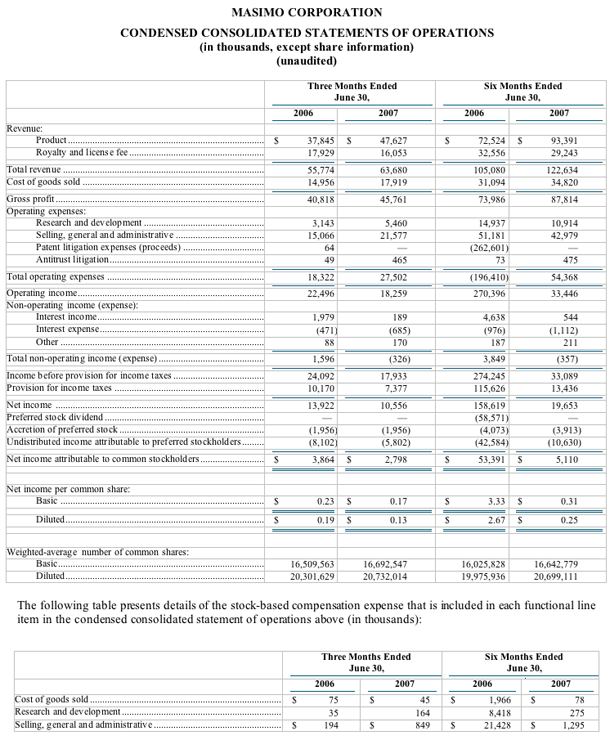
MASIMO CORPORATION
Unaudited Reconciliation of GAAP to Non-GAAP Earnings per Share
The following tables provide a comparison of our earnings per share calculated under Emerging Issues Task Force Issue No. 03-6, "Participating Securities and the Two-Class Method under FASB Statement No. 128", or EITF 03-6, in accordance with GAAP and the non-GAAP if converted method based upon Financial Accounting Standards Board No. 128 "Earnings per Share", or FASB 128. The non-GAAP if converted method assumes conversion of all shares of the Company's preferred stock into common stock as of December 31, 2006. As a result of the three-for-one forward stock split of our common stock effected on June 25, 2007, the conversion price of each outstanding share of our preferred stock was reduced to one-third of the pre-stock split conversion price, which effectively increased the conversion ratio to three shares of common stock for one share of preferred stock. Upon closing of our initial public offering on August 13, 2007, all outstanding shares of our preferred stock were converted into an aggregate of 34,612,503 shares of common stock. Therefore, effective August 13, 2007, the Company transitioned from computing earnings per share from the two class method, in accordance with EITF 03-6 to the if converted method in accordance with FASB 128. The Company believes that the following non-GAAP earnings per share information is relevant and useful information that can be used by analysts, investors and other interested parties to assess the Company's performance on a comparable basis to future reported earnings per share. Accordingly, the Company is disclosing this information to permit additional analysis of the Company's performance (in thousands, except share data):
Adjustments:
(1) Under the if converted method, no shares of preferred stock would have been outstanding, therefore no dividends would have been recorded specifically for preferred shareholders.
(2) Under the if converted method, no shares of preferred stock would have been outstanding; therefore no allocation of undistributed income to preferred stockholders would have been made.
(3) Under the if converted method, no shares of preferred stock would have been outstanding; therefore no accretion of preferred stock would have been recorded.
(4) Under the if converted method, all shares of preferred stock would have been outstanding of the entire period.


Masimo to Report Second Quarter 2007 Financial Results on September 19, 2007
Irvine, California, September 12, 2007 - Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced it will release second quarter financial results for the period ended June 30, 2007 after the market closes on Wednesday, September 19, 2007.
A conference call to review the results will begin at 2:00 p.m. PT (5:00 p.m. ET) and will be hosted by Joe E. Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President & Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the Company's corporate web site at www.masimo.com. The dial-in numbers are (866) 700-7441 for domestic callers and (617) 213-8839 for international callers. The reservation number for both dial-in numbers is 11107851. After the live webcast, the call will remain available on Masimo's web site through October 19, 2007. In addition, a telephonic replay of the call will be available until October 3, 2007. The replay dial-in numbers are (888) 286-8010 for domestic callers and (617) 801-6888 for international callers. Please use reservation code 65525346.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Masimo Corporation
Investors:
Mark P. de Raad
Executive Vice President and
Chief Financial Officer
(949) 297-7041
mderaad@masimo.com
Media:
Tom McCall
V.P. Marketing Communications
(949) 297-7075
tmccall@masimo.com


University of Wisconsin Hospitals and Clinics Complete System-Wide Conversion to Masimo SET Pulse Oximetry Technology
Irvine, California - September 6, 2007 - Masimo (NASDAQ:MASI) the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, announced the completion of the University of Wisconsin Hospital and Clinics' system-wide implementation of Masimo SET pulse oximetry. UW Hospitals and Clinics performed an extensive comparison and thorough evaluation spanning virtually every department and cited precise, continuous SpO2 measurements under demanding conditions as the chief reason for conversion to Masimo SET.
"We were looking for a product with accuracy in the face of difficult monitoring conditions such as patient movement. After evaluating multiple solutions for improving oximetry practice, we chose Masimo SET technology," said Christopher Green, M.D., Medical Director for the American Family Children's Hospital at the University of Wisconsin Hospitals and Clinics.
The University of Wisconsin Hospital and Clinics is a 471-bed facility that ranks among the finest academic medical centers in the United States. With more than 80 outpatient clinics and seven satellite locations, it is one of only two organizations in Wisconsin with designated Level One adult and pediatric trauma centers and is recognized as a national leader in fields such as cancer treatment, pediatrics, ophthalmology, surgical specialties and organ transplantation.
By making the conversion to Masimo, the University of Wisconsin Hospital and Clinics joins other top hospitals in the United States-including four of the top five-as listed on the US News & World Report Honor Roll, which have all adopted Masimo SET as their primary pulse oximetry platform. Masimo SET is widely recognized as the most accurate and reliable pulse oximetry technology in the world, clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 readings even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. These studies prove Masimo SET delivers improvements in outcomes, safety and efficiency.
In addition to the University of Wisconsin Hospitals and Clinics' system-wide standardization to Masimo SET pulse oximetry, the conversion also allows UW Hospitals and Clinics' clinicians to utilize Masimo Rainbow SET technology. Masimo Rainbow SET is an upgradeable noninvasive blood constituent monitoring platform that is capable of measuring additional blood constituents that previously required invasive procedures. Masimo Rainbow SET's first application is Pulse CO-Oximetry, the first and only technology platform capable of continuously and noninvasively measuring carboxyhemoglobin/carbon monoxide (SpCO), methemoglobin (SpMet), and Pleth Variability Index (PVI), in addition to oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate.
By quickly and accurately determining levels of SpCO and SpMet-two critical dyshemoglobins proven to increase morbidity and mortality in a broad range of clinical settings-clinicians can accurately determine their patients' true oxygenation status. This allows for more precise and timely diagnosis and treatment.
Pleth Variability Index (PVI) is a noninvasive measurement that quantifies changes in the plethysmographic waveform derived from pulse oximetry, and may provide clinicians with a noninvasive way to monitor functional hemodynamics in their patients. Clinicians who have evaluated PVI believe this technology will prove to be a valuable clinical tool with significant advantages over currently available indicators of changes in functional hemodynamics that are invasive, operator dependent, often inaccurate, and expensive.
Joe E. Kiani, Chairman and CEO of Masimo, stated, "We are proud to partner with The University of Wisconsin Hospitals and Clinics in their efforts to provide their patients with exceptional level of safety and care. This is clearly an organization that is focused on patients and understands the importance of adopting innovative technology to assist them in enhancing the quality of the care they deliver. The opening of the new American Family Children's Hospital, which is designed to give the best and kindest care possible, are examples of UW Hospitals and Clinics' commitment in this area. Their system-wide conversion to Masimo SET technology will further advance patient safety and care throughout this fine organization."
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corporation. ARM, Acoustic Respiratory Monitoring, BiFi, Rainbow, SpCO, SpMet, PVI, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corporation.


Apria Healthcare Chooses Masimo SET Pulse Oximetry for Use in the Company's Home Health Care Business
Ability of Masimo SET pulse oximetry technology to significantly reduce false alarms without missing true hypoxic events cited as the reason for choosing Masimo over all other technologies
Irvine, California August 22, 2007 - Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced a partnership with Apria Healthcare to provide pulse oximetry products and accessories for Apria's home health care business. Apria Healthcare is America's leading provider of integrated home health care products and services, offering a comprehensive range of home respiratory therapy, diabetic supplies, medications and equipment, home infusion therapy and home medical equipment services.
Apria conducted a competitive bid in which Masimo was selected due to the superior clinical performance of its proprietary Masimo SET pulse oximetry technology, which significantly enhances the ability of Apria's customers to detect hypoxic events in the home care setting. This will provide patients and caregivers with reliable warnings of these instances where blood oxygen levels begin to drop, which in turn will lead to expedient intervention and the delivery of the appropriate level of care.
Masimo SET has been clinically proven in more than 100 independent clinical and laboratory studies to provide the most trustworthy continuous noninvasive arterial blood oxygen saturation and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. These studies prove Masimo SET delivers improvements in outcomes, safety and efficiency by providing accurate measurement of arterial oxygen satuaration and pulse rate and significantly reducing false alarms while improving detection of true events.
Joe E. Kiani, Chairman & CEO of Masimo, stated, "We are happy that Apria has chosen Masimo SET technology to help keep their patients safe in a home health care environment and are excited to be partnering with the leading name in home health care. This partnership with Apria marks Masimo's entry into the home care market in the USA. Our mission has been to improve patient safety and reduce cost of care by taking noninvasive monitoring to new sites and applications. We are proud to form this partnership with Apria to bring reliable Masimo SET pulse oximetry into the home care setting."
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it substantially reduced false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical and laboratory studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corporation. ARM, Acoustic Respiratory Monitoring, BiFi, Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corporation.


Masimo Announces Closing of IPO and Exercise of Underwriters' Over-Allotment Option
Over-allotment option brings total offering amount to over $232 million
Irvine, California, August 13, 2007 - Masimo Corporation (NASDAQ: MASI), the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced that it has closed its previously announced initial public offering of 11,916,626 shares and that the underwriters of the offering have exercised their over-allotment option and purchased 1,787,494 additional shares of common stock at the public offering price of $17 per share. Including the over-allotment, the Company and selling stockholders sold 13,704,120 shares in the offering for gross proceeds of $232,970,040. Excluding shares offered by selling shareholders, net proceeds to the company amounted to approximately $48 million after deducting the underwriting discounts and commission and the estimated offering expenses.
Piper Jaffray & Co., Deutsche Bank Securities Inc. and Citigroup Global Markets Inc. acted as joint book running managers for the offering. Cowen and Company, LLC and Thomas Weisel Partners LLC, acted as co-managers of the offering. A copy of the final prospectus relating to the offering can be obtained from the Piper Jaffray & Co. Prospectus Department, phone (612) 303-6000.
The registration statement relating to the initial public offering of common shares has been declared effective by the Securities and Exchange Commission. This press release does not constitute an offer to sell or the solicitation of an offer to buy nor shall there be any sale of such common shares in any state in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET and with it substantially reduced false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical and laboratory studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements. All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our plans, objectives, strategies and prospects regarding, among other things, the financial condition, results of operations and business of ours and our subsidiaries. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control. Certain factors mentioned in this press release, including the risks outlined under "Risk Factors" in our Prospectus dated August 7, 2007, will be important in determining future results. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. They can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our Prospectus dated August 7, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Masimo Corporation
Investors:Mark de Raad
Executive Vice President and Chief Financial Officer
(949) 297-7041
mderaad@masimo.com
Media: Tom McCall
V.P. Marketing Communications
(949) 297-7075
tmccall@masimo.com
The Ruth Group
Nick Laudico
(646) 536-7030
nlaudico@theruthgroup.com


Masimo Prices Initial Public Offering
Irvine, California, August 8, 2007 - Masimo Corporation, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced the pricing of its initial public offering of 11,916,626 shares of common stock at a price of $17 per share, 10,416,626 of which are being sold by selling stockholders and the remainder by Masimo. Masimo has granted the underwriters an option to purchase up to an additional 1,787,494 shares of common stock to cover over-allotments, if any. Masimo will not receive any proceeds from the sale of shares by the selling stockholders.
Masimo intends to use the net proceeds from the offering for capital expenditures and the placement of equipment, sales and marketing activities to support the ongoing commercialization of the Masimo SET and Masimo Rainbow SET products, sales force expansion, research and development activities and including support of hardware and software product development and clinical study initiatives. The remainder of the net proceeds will be used for increased working capital and general corporate purposes.
Piper Jaffray & Co., Deutsche Bank Securities Inc. and Citigroup Global Markets Inc. acted as joint book running managers for the offering. Cowen and Company, LLC and Thomas Weisel Partners LLC, acted as co-managers of the offering.
Masimo Corporation common stock will trade on the NASDAQ Global Market under the symbol "MASI." The offering is made only by means of a prospectus, copies of which can be obtained from the Piper Jaffray & Co. Prospectus Department, phone (612) 303-6000.
The registration statement relating to the initial public offering of common shares has been declared effective by the Securities and Exchange Commission. This press release does not constitute an offer to sell or the solicitation of an offer to buy nor shall there be any sale of such common shares in any state in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET and with it substantially reduced false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical and laboratory studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations. Our Masimo SET platform has significantly addressed many of the previous technology limitations, has substantially contributed to improved patient outcomes and has been referred to by several industry sources as the gold standard in pulse oximetry. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements. All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our plans, objectives, strategies and prospects regarding, among other things, the financial condition, results of operations and business of ours and our subsidiaries. These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control. Certain factors mentioned in this press release, including the risks outlined under "Risk Factors" in our Prospectus dated August 7, 2007, will be important in determining future results. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. They can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the risk factors contained in our Prospectus dated August 7, 2007, whether as a result of new information, future events or otherwise, except as may be required under the federal securities laws.
Masimo Corporation
Investors:
Mark de Raad
Executive Vice President and
Chief Financial Officer
(949) 297-7041
mderaad@masimo.com
Media:
Brad Langdale
Chief Marketing Officer
(949) 297-7009
blangdale@masimo.com
The Ruth Group
Nick Laudico
(646) 536-7030
nlaudico@theruthgroup.com


Masimo Announces FDA Clearance for Additional Neonatal Parameters for Rainbow SET, Including Continuous and Noninvasive Measurement of Methemoglobin
Masimo Rainbow SET will now be available to monitor methemoglobin levels of neonatal patients undergoing inhaled nitric oxide (iNO) therapy for hypoxic respiratory failure
Irvine, California August 1, 2007 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced that it has received FDA 510(k) clearance for pulse oximetry (SpO2) and methemoglobin (SpMet) measurement in neonatal patients with its Rainbow adhesive sensors. The ability to continuously and noninvasively measure methemoglobin levels in neonatal patients is especially important in light of the use of inhaled nitric oxide (iNO) therapy to treat hypoxic respiratory failure in newborns, which has been shown to induce methemoglobinemia.
According to the American Academy of Pediatrics, "infants who receive iNO therapy should be monitored according to institutionally derived protocols designed to avoid the potential toxic effects associated with iNO administration. These effects include methemoglobinemia (secondary to excess nitric oxide concentrations), direct pulmonary injury (attributable to excess levels of nitrogen dioxide), and ambient air contamination".1
Masimo Rainbow SET provides clinicians with what is believed to be the only way to continuously and noninvasively measure methemoglobin levels in the blood (SpMet), making it an appropriate technology to incorporate into neonatal iNO therapies.
With the FDA clearance of SpO2 and SpMet in neonates, more patient populations can now benefit from the noninvasive and continuous measurements that Masimo Rainbow SET can provide, including the delivery of accurate, reliable SpO2 saturation measurements from 60%-100% even during the most difficult clinical conditions of motion and low perfusion.2 In addition to the neonatal clearance of SpMet and SpO2, the company announced improved accuracy specifications for the noninvasive measurement of carboxyhemoglobin (SpCO) in adult, pediatric and infant patients with its Masimo Rainbow SET technology using adhesive sensors, from + 3.5% to + 3%.
"We are happy to be able to extend the range of parameters of Masimo Rainbow SET technology available to clinicians treating neonatal patients and to be able to continue to improve the accuracy of our technology," explained Masimo Chairman and CEO Joe E. Kiani. "Attaining this clearance for SpMet in the neonatal population is the latest example of our commitment to providing enhanced levels of patient care in this critical patient population."
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET and with it substantially reduced false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical and laboratory studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the industry-leading in pulse oximetry and substantially contributing to improved patient outcomes. In 2005, Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
1 AMERICAN ACADEMY OF PEDIATRICS: Pediatrics, Vol. 106 No. 2 August 2000, pp. 344-345, Use of Inhaled Nitric Oxide, Committee on Fetus and Newborn
2 When used with Masimo Rainbow SET technology monitors or with licensed Masimo Rainbow SET technology modules using Rainbow series patient cables, during no motion, the accuracy of the Rainbow adhesive sensors in the range from 60% to 80% SpO2 is + 3 digits (+ 1 Std. Dev.) for adults/pediatrics/infants and 70% to 100% SpO2 is + 2 digits (+ 1 Std. Dev.) for adults/pediatrics/infants /neonates. The accuracy during motion (from 70% to 100%) is + 3 digits (+ 1 Std. Dev.) for adults/pediatrics/infants/neonates.
SpO2 and SpMet accuracy was determined on 16 neonatal NICU patients ranging in age from 7 to 135 days old and weighing between 0.5 and 4.25 kgs. Seventy-nine (79) data samples were collected over a range of 70% to 100% SaO2 and 0.5 - 2.5% SbMet with a resultant accuracy of 2.9% SpO2 and 0.9% SpMet.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corporation. ARM, Acoustic Respiratory Monitoring, BiFi, Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corporation.


Texas Society for Respiratory Care Honors Masimo for "Groundbreaking Innovation of Rainbow SET Technology" and "Continued Unwavering Support of the TSRC and the Respiratory Care Profession"
Masimo CEO Joe Kiani accepts award at the Texas Society for Respiratory Care's
36th Annual Meeting and Exposition in Austin, Texas.
Irvine, California June 28, 2007 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today received the first ever Texas Society for Respiratory Care (TSRC) LoneStar Award for Innovation and Support "for the groundbreaking innovation of Rainbow SET Technology, and the continued unwavering support of the TSRC and the Respiratory Care profession." Masimo Chairman and CEO, Joe Kiani, accepted the award on behalf of Masimo employees worldwide at the TSRC's 36th Annual Meeting and Exhibition in Austin, Texas.
"Masimo has advanced patient care with its read-through motion pulse oximetry technology, Masimo SET, making pulse oximeters a clinically useful tool," said TSRC President-Elect Terry Gilmore, MA, and RRT. "With their introduction of Masimo Rainbow SET and with it the noninvasive measurement of carboxyhemoglobin and methemoglobin, they are again advancing patient care by allowing respiratory care professionals to have a better understanding of the true oxygenation status of their patients' blood." Gary Herrin, MBA, Executive Director of the TSRC added that "we greatly appreciate Masimo's long standing support of the Respiratory Care profession and the TSRC, and we welcome the opportunity to enhance this partnership in patient care. We encourage their development and implementation of further groundbreaking technology"
"We are honored to receive this award from TSRC for the advances in patient care made possible by Masimo technologies, and are happy that respiratory care professionals continue to look to Masimo to help them provide cost-effective quality cardiopulmonary patient care." Mr. Kiani explained. "For nearly 20 years, we have been privileged to work alongside so many dedicated respiratory care professionals who shared our vision of always doing what is best for patient care. This award is very meaningful to us because it comes from an organization and a group of professionals we greatly admire."
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the industry leading pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. ARM, Acoustic Respiratory Monitoring, BiFi, Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corp.



Masimo Announces Continuous Noninvasive Functional Hemodynamic Monitoring With Plethysmograph Variability Index (PVI), Latest Masimo Rainbow SET Measurement
Symposium presentation during the 2007 European Society of Anaesthesia Congress in Munich, Germany cites independent research showing the potential value of this new noninvasive diagnostic tool
Irvine, California June 20, 2007 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, reported that new independent studies presented at a symposium during the European Society of Anaesthesia in Munich, Germany last week indicated that Masimo's Plethysmograph Variability Index (PVI), a noninvasive measurement that quantifies changes in the plethysmographic waveform derived from pulse oximetry, can provide clinicians with a noninvasive way to monitor functional hemodynamics in their patients.
Clinicians who have evaluated PVI believe this technology will prove to be a valuable clinical tool with significant advantages over currently available indicators of changes in functional hemodynamics that are invasive, operator dependent, often inaccurate, and expensive. PVI displays a numeric representation of the changes to the pleth waveform on the pulse oximeter and allows clinicians to track and trend these changes over time as well as a Diagnostic Plethysmograph that maintains the morphology of the true pleth for clinicians to view.
In his presentation in Munich, Maxime Cannesson, MD from the Claude Bernard University and Louis Pradel Hospital in Lyon, France cited research he co-authored entitled Ability of a Novel Algorithm for Noninvasive Automatic Estimation of the Respiratory Variations in the Pulse Oximeter Waveform to Detect Changes in Ventricular Preload scheduled for publication in the journal Anesthesiology later this year that indicates Masimo's PVI method of quantifying changes in ventricular preload, and therefore the ability of the heart to pump adequate blood to the tissues, correlated well with invasive methods.1
Dr. Cannesson said the authors had demonstrated in an earlier study that a change in the beat to beat amplitude of the oximeter waveform of greater than 15% predicted whether a patient was able to adapt to fluid restoration with an appropriate physiological response.2
Dr. Cannesson said that it was his belief that "Pleth Variability Index can automatically and noninvasively detect changes in ventricular preload in mechanically ventilated patients in the operating room. PVI shows great promise for use in perioperative fluid optimization which will have both clinical and economical impact. Other clinical applications for PVI are in the areas of fluid depletion/restriction, mechanical ventilator settings/adjustments, detection of changes in myocardial contractility."
Other clinicians have concurred with these observations and feel that the ability of Masimo Rainbow SET oximeters to display PVI may provide them with a useful clinical data point in the management of their patients.
Dr. Mitchell Goldstein of Loma Linda University Children's Hospital said "Trending of PVI may be useful in monitoring critical care and surgical patients, both intraoperatively and postoperatively, for appropriate hydration and ventilation status."
Dr. Dan Redford of the University of Arizona, University Medical Center added "PVI may be useful in monitoring surgical patients, for appropriate intravascular volume status. For example, a rising PVI may indicate developing hypovolemia, and a falling PVI post-fluid resuscitation is evidence of an appropriate fluid responsiveness, and important measure in the critical care/OR environment."
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the "gold standard" pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
1 "Ability of a Novel Algorithm for Noninvasive Automatic Estimation of the Respiratory Variations in the Pulse Oximeter Waveform to Detect Changes in Ventricular Preload", Maxime Cannesson, Bertrand Delannoy, Antoine Morand, Pascal Rosamel, Jean-Jacques Lehot. Anesthesiology 2007 (In Press).
2 "Relation Between Respiratory Variations in Pulse Oximetry Plethysmographic Waveform Amplitude and Arterial Pulse Pressure in Ventilated Patients", Maxime Cannesson, Cyril Besard, Pierre G. Durand, Julien Bohe, and Didier Jacques. Critical Care 2005.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. ARM, Acoustic Respiratory Monitoring, BiFi, Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corp.


Breaking Multi-Center Study: Masimo SET Pulse Oximetry Technology Shown to Significantly Lower Instances of Severe Retinopathy of Prematurity in Extremely Low Birth Weight Infants
Study released at the Pediatric Academic Societies' Annual Meeting this month in Toronto shows that switching to Masimo SET pulse oximetry technology can reduce the risk associated with ROP by 40%
Irvine, California May 30, 2007 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, reported that a new independent study presented at the Pediatric Academic Societies' (PAS) Annual Meeting in Toronto, Canada earlier this month showed that switching to Masimo SET pulse oximetry technology can reduce the risk of extremely low birth weight (ELBW) infants developing retinopathy of prematurity (ROP) by 40%.1 One of the most common causes of visual impairment and blindness in premature infants, ROP has been shown to be exacerbated by hyperoxia caused by the excessively high levels of supplemental oxygen often used to treat these patients, due in large part to unreliable oxygen level measurements from pulse oximeters not able to maintain accuracy during conditions of motion and low perfusion.
In the study entitled Clinical Practice and SpO2 Technology in the Prevention of ROP in ELBW Infants, a team of neonatologists from hospitals in Georgia, Texas and New Jersey headed by Armando Castillo, Richard Deulofeut, and Augusto Sola examined whether a change in pulse oximetry (SpO2) technology could impact treatment effect and relative risk reduction of ROP in ELBW infants. "The impact of (differences in pulse oximetry technologies) in reducing ROP is not easy to discriminate" the researchers stated in the study. "Infants under our care were exposed to a unique situation, where a universal change in clinical practice was implemented by the same health care team members, but SpO2 technology after the clinical change was not uniform."
As a result, the research team was able to analyze ROP rates before and after identical clinical practice changes at two different Health Care Centers and isolate the difference made by the pulse oximetry technology. The clinical changes included increased education and commitment of bedside care givers along with guidelines to decrease hyperoxemic periods and wide changes in oxygenation. Nellcor N395 pulse oximeters, with claims of accuracy during motion and low perfusion, were used in both Centers prior to the clinical practice changes, while after these changes Center #1 switched to Masimo SET pulse oximetry and Center #2 kept the Nellcor technology.
In Center #1, pre-change rates from 2000-2002 for ROP (III and IV) and laser treatments were 11.1% and 4% respectively, decreasing to 6% and 2.5% in 2003-2004 after the change in clinical practice and switch to Masimo SET-representing a 40% relative risk reduction in severe ROP. For the same time periods in Center #2, ROP (III and IV) and laser treatment rates remained unchanged at 13% and 5% respectively after changing clinical practice and maintaining use of Nellcor N395 technology, leading researchers to state that the results were "significantly more favorable" for the center that switched to Masimo technology.
"In a large group of examined inborn infants <1,250 grams treated by the same neonatologists, MDs and NNPs using the same clinical guideline to decrease hyperoxemia and wide changes in oxygenation, the relative risk rate of severe ROP and laser therapy are associated with the SpO2 technology utilized," the researchers concluded. "This further supports the significance of adequate SpO2 monitors in managing critically ill infants."
Joe E. Kiani, Chairman and CEO of Masimo stated; "In 2002, a group of researchers at Cedars Sinai led by Dr. Sola published a ground-breaking study, which showed that a new oxygen titration protocol, including the monitoring of the neonates' arterial blood oxygen saturation with Masimo SET pulse oximetry, could dramatically reduce ROP. But some observers questioned how critical Masimo SET pulse oximetry was to the success of the protocol. This multi-center study shows that the accuracy and reliability of Masimo SET pulse oximetry, in the hands of caring clinicians with sound protocol, is indeed important to achieving dramatic reductions in ROP."
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the industry leading pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
1 Clinical Practice and SpO2 Technology in the Prevention of ROP in ELBW Infants. Armando R. Castillo, Richard Deulofeut, Augusto Sola. Publication 8440.7; 2007 Pediatric Academic Societies'
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. ARM, Acoustic Respiratory Monitoring, BiFi, Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corp.


Masimo SET Pulse Oximetry Expanded into Welch Allyn Patient Monitoring Devices
Spot Vital Signs ® and Propaq ® LT Devices Extend Masimo SET Read-Through Motion SpO2 Monitoring Capabilities to More Patients in More Settings
Skaneateles, NY, May 16, 2007 - Welch Allyn, a leading global manufacturer of frontline medical products and solutions, and Masimo Corporation, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, have partnered to incorporate Masimo SET pulse oximetry into new versions of the Welch Allyn Spot Vital Signs and Welch Allyn Propaq LT devices. The two devices allow clinicians to effectively spot check and monitor patients in multiple acuity settings within the hospital and can be easily configured for efficient, safe and accurate operation by caregivers with varying levels of training and sophistication.
Masimo SET is the most accurate and reliable pulse oximetry technology in the world, clinically proven in more than 100 independent and objective studies to provide the most trustworthy arterial blood oxygen saturation and pulse rate measurements even under the most difficult clinical conditions, including patient motion and low peripheral perfusion. These studies prove Masimo SET delivers improvements in outcomes, safety and efficiency while decreasing costs.
"We are very pleased to extend our current business relationship with Masimo as we strive to offer our customers the safest and most accurate monitoring technologies available," said Doug Linquest, executive vice president, Monitoring and Defibrillation at Welch Allyn. "By continuing to integrate Masimo SET pulse oximetry technology into our extensive line of spot-check and monitoring systems, we are able to give caregivers the capability to monitor SpO2 in more patients in places they never thought possible."
Spot Vital Signs is a fully automated, multiparameter spot-check device that captures non-invasive blood pressure measurements using the oscillometric method. In addition to a pulse oximetry option, it also offers Welch Allyn's SureTemp® fast predictive thermometry which gives providers the option of performing oral, axillary or rectal temperature readings. Spot Vital Signs can also transfer data from the device into patients' medical records via an infrared connection designed to interface with the Welch Allyn Connex™ Data Management System.
The Propaq LT patient monitor can be used in a number of bedside, transport and ambulatory applications. It is deployable in areas in and outside the hospital, and comes equipped to monitor heart rate, 3- or 5-lead ECG, non-invasive blood pressure, SpO2, and respiration with an easy-to-use interface. It features a full-color LCD screen and can be configured for use on neonatal, pediatric and adult patients. The Propaq LT can be operated in stand-alone mode or wirelessly networked to Welch Allyn's Acuity Central Monitoring Station, and it can easily download data to a PC and customer Electronic Medical Records (EMR.)
"One of Welch Allyn's top priorities is to bring patient monitoring to the presently unmonitored bed, which makes up over eighty percent of today's hospital beds," added Linquest. "These beds are typically general care or medical-surgical beds and require advanced and connected spot-check vital signs devices. The new Propaq LT monitor effectively bridges the gap (cost, size and complexity) between today's continuous patient monitors and spot-check devices like Spot Vital Signs that offer even more comprehensive clinical benefits to the unmonitored bed."
"This partnership confirms the strength of our business relationship with Welch Allyn and the commitment of both companies to bring best in class patient monitoring technology to new clinical sites," said Joe E. Kiani, Chairman and CEO of Masimo Corporation. "We are happy that by partnering with Welch Allyn, we can offer the proven benefits of Masimo SET pulse oximetry to more clinicians and patients in more diverse clinical settings."
For more information about Welch Allyn vital signs capture and monitoring devices, please call 800.535.6663 or visit www.welchallyn.com.
About Welch Allyn
Welch Allyn, Inc. was founded in 1915 and is today a leading manufacturer of innovative medical diagnostic and therapeutic devices, cardiac defibrillators, patient monitoring systems, and miniature precision lamps. Headquartered in Skaneateles Falls, New York, USA, Welch Allyn employs more than 2,300 people and has numerous manufacturing, sales, and distribution facilities located throughout the world. Additional information on Welch Allyn and its products may be found at www.welchallyn.com.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the industry leading pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact Welch Allyn:
Jamie Arnold
Public Relations Manager
315.685.4599
arnoldj@welchallyn.com
Contact Masimo:
Tom McCall
Masimo Corporation
949-297-7075
tmccall@masimo.com


Masimo Bioacoiustic Respiratory Technology Featured On Front Page of Anesthesiology News
The April 2007 issue of Anesthesiology News included a front page feature on Masimo's new bioacoustic respiration technology. The article's headline said that Masimo's "Novel Respiratory Sensor May Find Wide Use in Hospitals, Noninvasive Device Could Be Key To Preventing Opioid-Related Deaths." The article went on to say that "A new acoustic monitor of respiratory rate appears to be as accurate as capnometry and is no more invasive than a Band-Aid."
Or the full article. Click the link below:
http://www.anesthesiologynews.com/index.asp?ses=ogst§ion_id=8&show=dept&article_id=7207


New Research Shows Masimo Acoustic Respiratory Monitoring Technology Provides "Significantly More Reliable Monitoring of Respiration Rate"
Irvine, California April 5, 2007 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, reported that three new independent studies, including one presented the recent International Anesthesiology Research Society (IARS) Clinical & Scientific Congress in Orlando, concluded that Masimo Acoustic Respiratory Monitoring technology (ARM) is "at least as accurate as capnometry" and "significantly more reliable" for monitoring respiration in spontaneously breathing patients.
Respiration is one of the five vital signs, but clinicians have long looked for a continuous and noninvasive method of monitoring respiration that is both clinically accurate, easy to use, and well tolerated by patients. Current methods of respiration monitoring, including impedance pneumography with ECG and end-tidal CO2 with capnometry, each have limitations that make them unreliable in certain clinical situations.
In the study released at the recent IARS, conducted by M. R. Macknet, MD and a team of researchers at Loma Linda University's Department of Anesthesiology, fifteen pediatric PACU patients were monitored with Masimo's ARM technology consisting of an adhesive bioacoustic sensor applied to the patient's neck and connected to a breathing frequency monitor prototype. In addition, a nasal cannula was placed, secured with tape, and connected to a capnometer. The study showed that "premature cannula dislodgement occurred in 14 patients" in less than 20 minutes, while "in no patient was the bioacoustic sensor dislodged before the end of the stay in the PACU." 1
"This data shows the relative ease and high incidence of capnometer cannula dislodgement compared to the new bioacoustic sensor," the researchers commented. "In clinical settings where continuous and reliable monitoring of spontaneous respiration is important, the new bioacoustic sensor provides significantly greater patient connection time, which should lead to significantly more reliable monitoring of respiration rate."
In two studies released in January at the 2007 Society for Technology in Anesthesiology annual meeting, researchers monitored ten postoperative adult ICU and six pediatric PACU patients in separate studies with Masimo's ARM technology as well as a nasal cannula connected to a capnometer. They concluded that Masimo's new bioacoustic respiratory sensor "demonstrates accuracy for respiratory rate monitoring as good as capnometry" and that the device "offers multiple benefits over existing devices and has a potential to improve monitoring in a general care setting." 2,3
Joe E. Kiani, Founder & CEO of Masimo stated, "We are happy to see that these initial independent studies reinforce our belief that Masimo Acoustic Respiratory Monitoring has the potential to be a breakthrough continuous and noninvasive respiratory rate monitoring technology that is accurate, reliable and easy to use. Similar to Masimo SET Read-Through Motion and Low Perfusion pulse oximetry technology, we recognized that one of the other five primary vital signs had no available method of giving reliable results and decided to see if we could come up with a better solution. We are encouraged that the initial clinical research indicates that the non-obtrusive nature of the Masimo ARM technology application may help overcome the problems of conventional respiratory rate monitors without compromising measurement accuracy."
Masimo expects to combine its Masimo Rainbow SET Read-Through Motion and Low Perfusion pulse oximetry with Masimo ARM technology as part of a general floor monitoring solution designed to increase patient safety and heed the growing call to find ways reduce unnecessary deaths on general care floors. The combination of these two technologies will give hospitals a continuous and noninvasive way to accurately monitor a patient's oxygenation and ventilation during patient-controlled analgesia, consistent with the new recommendations from the Anesthesia Patient Safety Foundation (APSF). In addition, the combination of Masimo Rainbow SET pulse oximetry and Masimo ARM should assist hospitals in being compliant with new American Society of Anesthesia (ASA) guidelines for management of patients at risk of obstructive sleep apnea (OSA) by providing an accurate and reliable combination of oxygen saturation and respiration rate monitoring.
The technology is currently undergoing additional clinical and engineering testing and is expected to be introduced at Alpha Sites in late 2007.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the industry leading pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
1 An improved sensor for monitoring respiration in pediatric patients. M. Macknet MD, P. Kimball-Jones MD, R. Applegate II MD, R. Martin MD, M. Allard M.B.Ch.B FRCA, Loma Linda University, Department of Anesthesiology
2 Accuracy of a novel bioacoustic sensor in adult postoperative patients. M. Macknet MD, P. Kimball-Jones MD, R. Applegate II MD, R. Martin MD, M. Allard M.B.Ch.B FRCA, Loma Linda University, Department of Anesthesiology
3 Accuracy of a novel bioacoustic sensor in pediatric postoperative patients. Macknet MD, P. Kimball-Jones MD, R. Applegate II MD, R. Martin MD, M. Allard M.B.Ch.B FRCA, Loma Linda University, Department of Anesthesiology
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. ARM, Acoustic Respiratory Monitoring, BiFi, Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corp.


Atom Medical to Incorporate Masimo Rainbow SET Technology into Atom's Neonatal and Premature Infant Product Lines
Masimo Rainbow SET is the first and only way for clinicians to continuously and noninvasively monitor their patients' carboxyhemoglobin, methemoglobin, and oxyhemoglobin saturation as well as pulse rate and perfusion index
Irvine, California -March 20, 2007- Atom Medical Corporation today announced that it has entered into a new agreement with Masimo that will allow it to incorporate Masimo Rainbow SET technology into its neonatal and premature infant product lines. Masimo Rainbow SET is a breakthrough noninvasive blood constituent monitoring platform that has the promise of measuring many blood constituents that previously required invasive procedures. Masimo Rainbow SET's initial application is Pulse CO-Oximetry, the first and only technology platform capable of continuously and noninvasively measuring carboxyhemoglobin (SpCO) and methemoglobin (SpMet), in addition to oxyhemoglobin (SpO2), perfusion index (PI), Pleth Variability Index (PVI) and pulse rate.
Masimo Rainbow SET uses multiple (7+) wavelengths of light housed in a single, simple-to-apply sensor to accurately determine levels of SpCO and SpMet-two critical dyshemoglobins, that if not monitored and treated, has proven to increase morbidity and mortality in a broad range of clinical settings. This gives clinicians the ability to accurately determine their patients' true oxygenation status, allowing for more precise and timely diagnosis and treatment.
Built on Read-Through Motion and Low Perfusion Masimo SET technology, Masimo Rainbow SET has been designed as an upgradeable technology platform. Masimo scientists are currently at work using the data delivered by Rainbow's multiple wavelengths of light to qualify additional noninvasive parameters, such as total hemoglobin (SpHb), which are anticipated to be able to be field-installed on Rainbow-ready monitoring devices through a simple software upgrade, so clinicians can easily purchase and add them when they become available.
Barry Matsubara, Chairman and Chief Executive Officer of Atom stated; "We have enjoyed a long partnership with Masimo. The main goal of this partnership has been to improve the care of patients our products touch. We entered into this partnership with Masimo nearly 10 years ago because we believed Masimo had made a tremendous stride with its Masimo SET Read-Through Motion and Low Perfusion technology. Today, Masimo SET is clearly considered the gold standard in pulse oximetry and has been shown to improve patient care across the globe. We believe that the new Rainbow Technology will have similar impact, with the ability to measure carboxyhemoglobin and methemoglobin noninvasively. We are looking forward to being one of the first companies to provide our customers with this life saving monitoring technology."
Joe E. Kiani, Chairman and CEO of Masimo stated; "We are very happy to expand our long and productive relationship with Atom to bring Masimo Rainbow SET's new monitoring capabilities to neonatal and infant care-where Atom excels. This has always been an important segment of the market for Masimo. Not only because they are the most challenging patient population, but because having the ability to make an impact on neonates' lives provides a longer benefit than for any other patient population. We continue to focus our efforts heavily in neonatal and infant monitoring as evidenced by innovations like our Blue sensor, which is specially designed to monitor cyanotic babies with very low oxygen saturation and children with congenital heart defects, and our Newborn sensor designed for newborn resuscitation and examination."
About Atom Medical Corporation
Atom Medical has an active and strong sales network is made up of over 50 overseas distributors who market, sell, and service Atom's name brand perinatal products to hospitals and clinicians in over 90 countries. In addition, long term relationships with over ten international medical manufacturers ensure that local clinical needs can be met with the latest imported medical products. Atom Medical will continue its commitment to providing the finest in medical products and care to its worldwide customers.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the industry leading pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Tom McCall, Masimo Corporation
Phone: 949-297-7075
Email: tmccall@masimo.com
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow, SpCO, SpMet, SpHb, PVI, and Pulse CO-Oximeter are trademarks of Masimo Corp.


Breaking Study: Masimo Blue Sensor Shown to be Most Accurate in Monitoring Cyanotic Infants
Study presented at the Society for Critical Care Medicine's 2007 annual meeting concludes Masimo Blue sensor superior in continuous and accurate pulse oximetry monitoring of cyanotic infants with congenital heart disease
Irvine, California -March 6, 2007- Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion Pulse Oximetry, reported that an independent study presented at the 2007 Society for Critical Care Medicine (SCCM) Annual Meeting in Orlando clearly demonstrated the superiority of Masimo LNOP Blue Sensors in providing accurate, reliable and continuous pulse oximetry readings in cyanotic infants with congenital heart disease. In the study, the Masimo LNOP Blue sensor-which was specifically designed for use in pediatric patients with low oxygen saturation-was compared to a Nellcor OxiMax sensor and to a standard Masimo LNOP sensor not designed for cyanotic patients, and was shown to have a "significantly higher correlation to arterial blood gas in comparison to the two other sensors studied." 1
The study was performed by a team of researchers from the Department of Clinical Engineering and Anesthesiology at St. Mary's Hospital in Fukuoka, Japan, who stated that it is extremely difficult to measure low saturation values (<70%) with accuracy with a conventional pulse oximeter. They explained that infants with congenital heart disease (CHD) are often kept at low oxygen saturation levels in order to maintain cardiac output and perfusion and that because of this, it is especially important for clinicians to be able to accurately measure their oxygen saturation by pulse oximeter.
To determine the accuracy of the Masimo Blue sensor with this difficult patient population, researchers analyzed 45 arterial blood gas samples received from six cyanotic infants undergoing pulmonary artery banding procedures and compared pulse oximetry data with arterial blood samples analyzed by CO-Oximetry. The results clearly showed the Masimo LNOP Blue sensor delivered more accurate and reliable readings, with a bias (indicating 95% limit agreement) and precision of 0.83 and 2.16 respectively, compared to 8.24 and 4.23 for the Nellcor OxiMax and 7.83 and 4.57 for the standard LNOP. The root-mean-square-a method of analyzing bias and precision-of these results shows that the Masimo LNOP Blue sensor is four times more accurate than the other sensors.
The researchers reported, "The LNOP Blue Sensor indicated significantly higher correlation to arterial blood gas in comparison to other two sensors studied. Therefore, LNOP Blue sensor can be a solution for monitoring accurately low saturation levels. Accurate non-invasive monitoring can reduce the number of arterial blood gas draws and can also reduce the risk of infection related to blood draws. The LNOP Blue Sensor provides for the accurate measurement of pulse oximetry" when used on cyanotic infants with congenital heart disease.
Joe E. Kiani, Chairman and CEO of Masimo stated, "We are happy to see that researchers all around the world are recognizing and validating the effectiveness of our LNOP Blue sensor with Masimo SET technology in providing accurate, reliable and continuous pulse oximetry readings in infants with congenital heart disease, enabling clinicians to more effectively care for one of the most vulnerable patient populations. With this study, there have now been a total of five independent studies on the LNOP Blue sensor conducted in the United States, Japan and Canada-all with similar results. At Masimo, we take very seriously our legacy of developing technologies that enable more advanced and comprehensive care for those patients who are most at risk. We will continue to expand and deepen our technology portfolio to provide a broad spectrum of noninvasive monitoring parameters that help caring clinicians make better diagnostic and treatment decisions across a wide range of patient populations."
1 Clinical evaluation of Accuracy of Masimo LNOP Blue sensor in cyanotic infants: Yoshimitsu Tsutsumi, Masakazu Nakashima, Takeshi Ifuku, Hiroshi Yasunaga M.D., Fumiya Nakao M.D., Jun Takamatsu M.D. Department of Clinical Engineering and Anesthesiology, St. Mary's Hospita, Fukuoka, Japan
-end-
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the industry leading pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Tom McCall, Masimo Corporation
Phone: 949-297-7075
Email: tmccall@masimo.com
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corp.


Masimo Expands $500,000 Performance Guarantee to Include All Commercially Available Pulse Oximetry Technologies
Masimo guarantees its Masimo SET pulse oximetry will outperform any other commercially available technology in a hospital evaluation or it will pay up to $500,000
Irvine, California February 7, 2007 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, today announced that it is expanding its $500,000 performance guarantee to include all other commercially available pulse oximetry technologies. If, after a side-by-side hospital evaluation, Masimo SET does not outperform any other commercially available pulse oximetry technology under the most challenging conditions of motion and low perfusion, Masimo will pay up to $500,000 towards the purchase of that pulse oximetry. This offer is available only to hospitals whose goal is to upgrade their pulse oximetry hospital-wide. Important details, conditions and qualifications for the guarantee are available on Masimo's web page: http://www.masimo.com.
Masimo SET is the most accurate and reliable pulse oximetry technology in the world under conditions of motion and low perfusion, clinically proven in more than 100 independent and objective studies to provide the most trustworthy SpO2 readings even under the most difficult clinical conditions. These studies prove Masimo SET delivers improvements in outcomes, safety and efficiency while decreasing costs.
"We are hopeful that decision makers within hospitals see this guarantee as a 'can't lose' offer," stated Joe E. Kiani, Founder and CEO of Masimo. "If the evaluation proves that their level of care can be improved with Masimo SET, not only will they benefit from improved patient outcomes and safety, but in most cases, the conversion can be completed with no capital outlay and no increase in annual operating costs. If the evaluation proves that Masimo is not the best performing, the hospital will receive $500,000 towards the purchase of another technology that can deliver similar patient care benefits."
Mr. Kiani continued: "We have converted hundreds of leading hospitals, including nearly two thirds of hospitals on the U.S. News and World Reports Honor Roll. The national drive to improve patient care and safety and reduce medical errors has been a primary driver for many of these hospitals' decision to evaluate and convert to Masimo SET technology. We are grateful that our products can play a role in this noble endeavor."
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the industry leading pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corp.


Independent Study Concludes Masimo Engineering Prototype is the First Technology to Measure Total Hemoglobin Continuously and Noninvasively, Wins STA Technology Award
Irvine, California January 25, 2007 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, reported that an independent study concluding that a preliminary Masimo engineering prototype can accurately measure total hemoglobin continuously and noninvasively has won the prestigious "Excellence in Technology Innovation" award at the 2007 Annual Meeting of the Society for Technology in Anesthesia (STA).
In the study, conducted by M. R. Macknet, MD and a team of researchers at Loma Linda University's Department of Anesthesiology, an engineering prototype from Masimo designed to measure total hemoglobin noninvasively was compared to invasive laboratory CO-Oximetry in its ability to accurately measure total hemoglobin levels in 19 patients scheduled to undergo surgery and nine healthy volunteers undergoing a hemodilution protocol. After reviewing 458 data pairs collected from the 28 patients, researchers concluded that the Masimo technology accurately delivered total hemoglobin levels, with the study showing bias, precision and ARMS of -0.039, 1.09, and 1.09 respectively when compared to invasive laboratory CO-Oximetry.1
Researchers concluded, "This device is the first device developed that can continuously and non-invasively measure hemoglobin concentration in addition to the other common hemoglobin species, and therefore provides a significant expansion to existing physiologic monitoring technology."
The same group of researchers from Loma Linda also presented a case study where the Masimo engineering prototype was used to track total hemoglobin during a liver and kidney transplant procedure. During the 16-hour procedure in which the Masimo device gave continuous readings, 17 blood samples were taken to correlate total hemoglobin levels. The researchers stated, "SpHb correlated well with CO-Oximeter determined Hb during most of this complicated procedure. Measurements showed good correlation during times of rapidly changing Hb concentration related to surgical blood loss and transfusion. Continuous and noninvasive hemoglobin monitoring would be an extremely useful tool in many clinical scenarios. This technology has the potential to greatly improve patient care and safety during surgical procedures."2
Masimo intends for this application to become part of the Rainbow SET platform. Masimo Rainbow SET is a platform which currently allows for the measurement of many blood constituents that have never before been measured noninvasively. Carboxyhemoglobin (SpCO) and methemoglobin (SpMet), as well as oxyhemoglobin (SpO2) that is accurate during motion and low perfusion, are currently commercially available to clinicians with Masimo Rainbow SET Radical-7. Extensive engineering and additional clinical research is currently underway to finalize the measurement of total hemoglobin with the plan to have SpHb ready for commercial use in 2007. Total hemoglobin content is one of the most frequently ordered lab tests and is critical to maintaining adequate oxygenation.
A noninvasive method of quantifying total hemoglobin, oxygen saturation, methemoglobin and carboxyhemoglobin would speed diagnosis and proper treatment of patients. According to the Loma Linda research team, "rapid measurement of hemoglobin would be an extremely useful tool in many clinical scenarios. This technology (Masimo Rainbow SET) in combination with methemoglobin and carboxyhemoglobin measurements should allow for significant advances in patient care."
Joe E. Kiani, Founder & CEO of Masimo stated, "This is the third time Masimo technology has won such a prestigious award from the Society of Technology in Anesthesia. In 1995, we won the Excellence in Technology Innovation award for the foundational technology of Masimo SET, the first pulse oximetry technology to provide accurate and reliable SpO2 measurements during motion and low peripheral perfusion. Last year Masimo Rainbow SET won the Application of Technology award for creating the first technology to provide noninvasive measurement of carboxyhemoglobin and methemoglobin levels in the blood, and this year we are honored with the Excellence in Technology Innovation award for our latest work on continuous and noninvasive hemoglobin monitoring."
"We thank the STA for encouraging and supporting our zeal for innovation for the betterment of patient care. We also extend our gratitude and congratulations to both our engineering team, and the independent clinical researchers, such as Dr. Mark Macknet. Without their passion for helping patients, we could not accomplish anything", continued Mr. Kiani.
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the industry leading pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
1 Continuous Non-Invasive Measurement of Hemoglobin via Pulse CO-oximetry. M. R. Macknet MD, S. Norton MD, P. Kimball-Jones MD, R. Applegate II MD, R. Martin MD, M. Allard M.B.Ch.B FRCA. Loma Linda University, Department of Anesthesiology
2 Continuous Non-Invasive Measurement of Hemoglobin via Pulse CO-oximetry During Liver Transplantation, a Case Report. M. R. Macknet MD, P. Kimball-Jones MD, R. Applegate II MD, R. Martin MD, M. Allard M.B.Ch.B FRCA. Loma Linda University, Department of Anesthesiology
Contact:
Tom McCall
Masimo Corporation
949-297-7075
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving Outcomes and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow, SpCO, SpMet, SpHb and Pulse CO-Oximeter are trademarks of Masimo Corp.


New Peer-Reviewed Clinical Study Concludes that Masimo Rainbow SET Technology is Accurate in Noninvasively Detecting Both Carbon Monoxide and Methemoglobin Poisoning
Irvine, California January 9, 2007 - Masimo, the inventor of Pulse CO-Oximetry and Read-Through Motion and Low Perfusion pulse oximetry, reported that results of a human volunteer study published in the peer-reviewed journal, Anesthesiology, concluded Masimo Rainbow SET technology accurately and reliably detects both carbon monoxide and methemoglobin poisoning. The study's authors said that the accuracy of this technology in measuring methemoglobin "is approximately the same as the specified uncertainty of the (invasive) laboratory CO-oximeters," adding that Masimo Rainbow SET technology "represents a significant improvement in our oxygenation monitoring capability."1
The new study was led by Steven J. Barker, PhD, MD, who is the Chair of the Department of Anesthesiology at the University of Arizona and has recently joined Masimo's Board of Directors. Ten volunteers breathed 500 ppm carbon monoxide until their carboxyhemoglobin (hemoglobin contaminated by carbon monoxide) levels reached 15%, and ten different volunteers received 300 mg of intravenous sodium nitrite to induce methemoglobin, a dysfunctional form of hemoglobin that starves tissues of oxygen. All subjects were instrumented with arterial cannulas and six Masimo Rainbow SET-enabled monitoring devices. Arterial blood was analyzed by three laboratory CO-Oximeters, and the resulting carboxyhemoglobin and methemoglobin measurements were compared with the corresponding pulse oximeter readings. The results showed the Masimo Rainbow SET technology measured carboxyhemoglobin with an uncertainty of 2% within the range of 0-15%, and it measured methemoglobin with an uncertainty of 0.5% within the range of 0-12%.
The University of Arizona researchers concluded that Masimo Rainbow SET technology "seems to be a major advance in pulse oximetry. It is the first commercially produced pulse oximeter to use multiple wavelengths of light, and we have found it to be capable of detecting and measuring both methemoglobin and carboxyhemoglobin."
The Arizona study reinforces clinical studies presented at last month's American Association of Respiratory Care (AARC) Congress in Las Vegas. Studies released at the AARC highlighted the significant clinical benefits to be gained by noninvasively screening patients for carbon monoxide poisoning using the "rapid," "inexpensive" and "reliable" Masimo Rainbow SET Pulse CO-Oximetry technology.
In one study released at the AARC, which included more than 5,000 patients, a research team led by Dr. Robert Partridge and Dr. Gregory Jay of Rhode Island Hospital at Brown University Medical School concluded that the use of Masimo Rainbow SET as a noninvasive test for carbon monoxide toxicity (COT) can effectively and efficiently be performed at ED triage, and that "unsuspected COT may be identified using noninvasive COHb screening and the prevalence of COT may be higher than previously recognized."2
Additionally, a group of researchers at the Erlanger Health System in Chattanooga, TN used the Masimo Rainbow SET technology to assess CO levels on 136 patients who presented to the outpatient pulmonary lab for arterial blood gas (ABG) draws to evaluate patient's smoking history as well as 21 patients who presented with burns and inhalation injuries in the ED who also received ABGs. They concluded that the technology was "quite reliable at detecting elevated CO levels in patients presenting to the pulmonary lab or emergency department."3
About Masimo
Masimo develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Read-Through Motion and Low Perfusion pulse oximetry, known as SET, and with it virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent clinical studies have confirmed that Masimo SET technology allows clinicians to accurately monitor blood oxygen saturation in critical care situations-establishing the technology as the industry leading pulse oximetry and substantially contributing to improved patient outcomes. In 2005 Masimo introduced Masimo Rainbow SET Pulse CO-Oximetry, which, for the first time, noninvasively monitors the level of carbon monoxide and methemoglobin in the blood, allowing early detection and treatment of potentially life-threatening conditions. Masimo, founded in 1989, has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Tom McCall
Masimo Corporation
949-297-7075
1 Measurement of Carboxyhemoglobin and Methemoglobin by Pulse Oximetry, A Human Volunteer Study: Steven J. Barker, Ph.D., M.D., Jeremy Curry, M.D., Daniel Redford, M.D., Scott Morgan, B.S.: Anesthesiology 2006; 105:1-1
2 Non-Invasive Carboxyhemoglobin Monitoring: Screening Emergency Department Patients for Carbon Monoxide Exposure. Partridge R, Chee KJ, Suner S, Sucov A, Jay G. Department of Emergency Medicine, Rhode Island Hospital, Brown Medical School, Providence, RI.
3 Evaluation of a New Pulse CO-Oximeter: Noninvasive Measurement of Carboxyhemoglobin in the Outpatient Pulmonary Lab and Emergency Departments. Layne T, Snyder C, Brooks D, Enjeti. Pulmonary Physiology Department, Erlanger Health System, Chattanooga, TN.
Masimo, SET, Signal Extraction Technology, Radical, Radical-7, Rad57, APOD, and Improving and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications are registered trademarks of Masimo Corp. Rainbow, SpCO, SpMet and Pulse CO-Oximeter are trademarks of Masimo Corp.
